Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): results from the AML Study Group
- PMID: 31554635
- PMCID: PMC9635584
- DOI: 10.1182/blood.2019001425
Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): results from the AML Study Group
Abstract
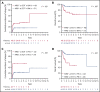
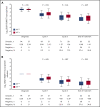
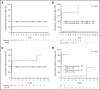
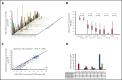
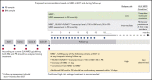
We performed serial measurable residual disease (MRD) monitoring in bone marrow (BM) and peripheral blood (PB) samples of 155 intensively treated patients with RUNX1-RUNX1T1+ AML, using a qRT-PC-based assay with a sensitivity of up to 10-6. We assessed both reduction of RUNX1-RUNX1T1 transcript levels (TLs) and achievement of MRD negativity (MRD-) for impact on prognosis. Achievement of MR2.5 (>2.5 log reduction) after treatment cycle 1 and achievement of MR3.0 after treatment cycle 2 were significantly associated with a reduced risk of relapse (P = .034 and P = .028, respectively). After completion of therapy, achievement of MRD- in both BM and PB was an independent, favorable prognostic factor in cumulative incidence of relapse (4-year cumulative incidence relapse: BM, 17% vs 36%, P = .021; PB, 23% vs 55%, P = .001) and overall survival (4-year overall survival rate BM, 93% vs 70%, P = .007; PB, 87% vs 47%, P < .0001). Finally, during follow-up, serial qRT-PCR analyses allowed prediction of relapse in 77% of patients exceeding a cutoff value of 150 RUNX1-RUNX1T1 TLs in BM, and in 84% of patients exceeding a value of 50 RUNX1-RUNX1T1 TLs in PB. The KIT mutation was a significant factor predicting a lower CR rate and inferior outcome, but its prognostic impact was outweighed by RUNX1-RUNX1T1 TLs during treatment. Virtually all relapses occurred within 1 year after the end of treatment, with a very short latency from molecular to morphologic relapse, necessitating MRD assessment at short intervals during this time period. Based on our data, we propose a refined practical guideline for MRD assessment in RUNX1-RUNX1T1+ AML.
© 2019 by The American Society of Hematology.
Figures

References
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. - PubMed
-
- Schlenk RF, Benner A, Krauter J, et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2004;22(18):3741-3750. - PubMed
-
- Marcucci G, Mrózek K, Ruppert AS, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23(24):5705-5717. - PubMed
-
- Prébet T, Boissel N, Reutenauer S, et al. ; Core Binding Factor Acute Myeloid Leukemia (CBF AML) intergroup . Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: a collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27(28):4747-4753. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

