The genetics of interstitial lung diseases
- PMID: 31554702
- PMCID: PMC9488931
- DOI: 10.1183/16000617.0053-2019
The genetics of interstitial lung diseases
Abstract
Interstitial lung diseases (ILDs) are a set of heterogeneous lung diseases characterised by inflammation and, in some cases, fibrosis. These lung conditions lead to dyspnoea, cough, abnormalities in gas exchange, restrictive physiology (characterised by decreased lung volumes), hypoxaemia and, if progressive, respiratory failure. In some cases, ILDs can be caused by systemic diseases or environmental exposures. The ability to treat or cure these ILDs varies based on the subtype and in many cases lung transplantation remains the only curative therapy. There is a growing body of evidence that both common and rare genetic variants contribute to the development and clinical manifestation of many of the ILDs. Here, we review the current understanding of genetic risk and ILD.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: R. Borie reports personal fees and non-financial support from Roche, and Boehringer Ingelheim, outside the submitted work. Conflict of interest: P. Le Guen has nothing to disclose. Conflict of interest: M. Ghanem has nothing to disclose. Conflict of interest: C. Taillé has received personal fees and other funding from AstraZeneca and Roche, personal fees from Teva and Genzyme, grants, personal fees and other from GlaxoSmithKline, Novartis and Sanofi, and other funding from Boehringer Ingelheim. Conflict of interest: C. Dupin reports personal fees, non-financial support and other from AstraZeneca, Boehringer, GlaxoSmithKline and Novartis, personal fees and other from Chiesi, personal fees from Sanofi, and non-financial support and other from Roche, outside the submitted work. Conflict of interest: P. Dieudé has nothing to disclose. Conflict of interest: C. Kannengiesser has nothing to disclose. Conflict of interest: B. Crestani reports personal fees from AstraZeneca, grants, personal fees and non-financial support from Boehringer Ingelheim and Roche, personal fees and non-financial support from Sanofi, and grants from Novartis, outside the submitted work.
Figures
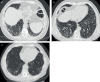

References
-
- Bjoraker JA, Ryu JH, Edwin MK, et al. . Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998; 157: 199–203. - PubMed
-
- Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 431–440. - PubMed
-
- King TE, Bradford WZ, Castro-Bernardini S, et al. . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, et al. . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. - PubMed
-
- Hutchinson J, Fogarty A, Hubbard R, et al. . Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J 2015; 46: 795–806. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
