Human PI3Kγ deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology
- PMID: 31554793
- PMCID: PMC6761123
- DOI: 10.1038/s41467-019-12311-5
Human PI3Kγ deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology
Abstract
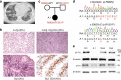
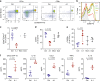
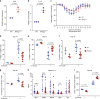
Phosphatidylinositol 3-kinase-gamma (PI3Kγ) is highly expressed in leukocytes and is an attractive drug target for immune modulation. Different experimental systems have led to conflicting conclusions regarding inflammatory and anti-inflammatory functions of PI3Kγ. Here, we report a human patient with bi-allelic, loss-of-function mutations in PIK3CG resulting in absence of the p110γ catalytic subunit of PI3Kγ. She has a history of childhood-onset antibody defects, cytopenias, and T lymphocytic pneumonitis and colitis, with reduced peripheral blood memory B, memory CD8+ T, and regulatory T cells and increased CXCR3+ tissue-homing CD4 T cells. PI3Kγ-deficient macrophages and monocytes produce elevated inflammatory IL-12 and IL-23 in a GSK3α/β-dependent manner upon TLR stimulation. Pik3cg-deficient mice recapitulate major features of human disease after exposure to natural microbiota through co-housing with pet-store mice. Together, our results emphasize the physiological importance of PI3Kγ in restraining inflammation and promoting appropriate adaptive immune responses in both humans and mice.
Conflict of interest statement
C.L.L. has received travel and speaker fees from Novartis. The remaining authors declare no competing interests.
Figures

References
-
- Fischer, A. & Rausell, A. Primary immunodeficiencies suggest redundancy within the human immune system. Sci. Immunol. 1, eaah5861 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

