Prolonged exposure to simulated microgravity diminishes dendritic cell immunogenicity
- PMID: 31554863
- PMCID: PMC6761163
- DOI: 10.1038/s41598-019-50311-z
Prolonged exposure to simulated microgravity diminishes dendritic cell immunogenicity
Abstract
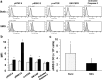
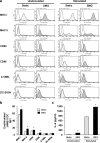
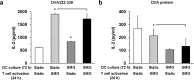
Immune dysfunction due to microgravity remains a hurdle in the next step of human space exploration. Dendritic cells (DC) represent a critical component of immunity, given their role in the detection of invaders and the subsequent task of activating T cells to respond and eliminate the threat. Upon encounter with microbes, DC undergo a process of maturation, whereby the cells upregulate the expression of surface proteins and secrete cytokines, both required for the optimal activation of CD4+ and CD8+ T cells. In this study, DC were cultured from 2-14 days in a rotary cell culture system, which generates a simulated microgravity (SMG) environment, and then the cells were assessed for maturation status and the capacity to activate T cells. Short-term culture (<72 h) of DC in SMG resulted in an increased expression of surface proteins associated with maturation and interleukin-6 production. Subsequently, the SMG exposed DC were superior to Static control DC at activating both CD4+ and CD8+ T cells as measured by interleukin-2 and interferon-γ production, respectively. However, long-term culture (4-14 d) of DC in SMG reduced the expression of maturation markers and the capacity to activate T cells as compared to Static DC controls.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Cogoli A. The effect of space flight on human cellular immunity. Environ. Med. 1993;37:107–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

