Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases
- PMID: 31555123
- PMCID: PMC6722391
- DOI: 10.3389/fnagi.2019.00232
Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases
Abstract
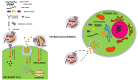
Aging is consistently reported as the most important independent risk factor for neurodegenerative diseases. As life expectancy has significantly increased during the last decades, neurodegenerative diseases became one of the most critical public health problem in our society. The most investigated neurodegenerative diseases during aging are Alzheimer disease (AD), Frontotemporal Dementia (FTD) and Parkinson disease (PD). The search for biomarkers has been focused so far on cerebrospinal fluid (CSF) and blood. Recently, exosomes emerged as novel biological source with increasing interest for age-related neurodegenerative disease biomarkers. Exosomes are tiny Extracellular vesicles (EVs; 30-100 nm in size) released by all cell types which originate from the endosomal compartment. They constitute important vesicles for the release and transfer of multiple (signaling, toxic, and regulatory) molecules among cells. Initially considered with merely waste disposal function, instead exosomes have been recently recognized as fundamental mediators of intercellular communication. They can move from the site of release by diffusion and be retrieved in several body fluids, where they may dynamically reflect pathological changes of cells present in inaccessible sites such as the brain. Multiple evidence has implicated exosomes in age-associated neurodegenerative processes, which lead to cognitive impairment in later life. Critically, consolidated evidence indicates that pathological protein aggregates, including Aβ, tau, and α-synuclein are released from brain cells in association with exosomes. Importantly, exosomes act as vehicles between cells not only of proteins but also of nucleic acids [DNA, mRNA transcripts, miRNA, and non-coding RNAs (ncRNAs)] thus potentially influencing gene expression in target cells. In this framework, exosomes could contribute to elucidate the molecular mechanisms underneath neurodegenerative diseases and could represent a promising source of biomarkers. Despite the involvement of exosomes in age-associated neurodegeneration, the study of exosomes and their genetic cargo in physiological aging and in neurodegenerative diseases is still in its infancy. Here, we review, the current knowledge on protein and ncRNAs cargo of exosomes in normal aging and in age-related neurodegenerative diseases.
Keywords: Alzheimer’s disease; Parkinson’s disease; aging; exosomes; frontotemporal dementia; non-coding RNA.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

