Antiviral Properties of R. tanguticum Nanoparticles on Herpes Simplex Virus Type I In Vitro and In Vivo
- PMID: 31555137
- PMCID: PMC6737004
- DOI: 10.3389/fphar.2019.00959
Antiviral Properties of R. tanguticum Nanoparticles on Herpes Simplex Virus Type I In Vitro and In Vivo
Abstract
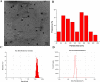
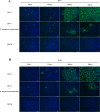
Herpes simplex virus type 1 (HSV-1), an enveloped DNA virus, plays a key role in varieties of diseases including recurrent cold sores, keratoconjunctivitis, genital herpes and encephalitis in humans. Great efforts have been made in developing more effective and less side-effects anti-herpes simplex virus agents, including traditional Chinese herbal medicines. In the present study, we evaluated the antiviral efficacy of Rheum tanguticum nanoparticles against HSV-1 in vitro and in vivo. R. tanguticum nanoparticles could inactivate the HSV-1 virions and block the viral attachment and entry into cells. Time-of-addition assay indicated that R. tanguticum nanoparticles could interfere with the entire phase of viral replication. Besides, R. tanguticum nanoparticles showed the ability to inhibit the mRNA expression of HSV-1 immediate early gene ICP4 and early gene ICP8 as well as the expression of viral protein ICP4 and ICP8. Moreover, R. tanguticum nanoparticles have been proved to protect mice against HSV-1 induced lethality by decreasing the viral load and alleviated pathological changes in brain tissues. In conclusion, we demonstrated that R. tanguticum nanoparticles could inhibit HSV-1 infection through multiple mechanisms. These results suggest that R. tanguticum nanoparticles may have novel roles in the treatment of HSV-1 infection.
Keywords: HSV-1; R. tanguticum nanoparticles; antiviral; mouse model; viral protein.
Figures

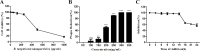
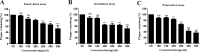



References
-
- Arokiyaraj S., Vincent S., Saravanan M., Lee Y., Oh Y. K., Kim K. H. (2017). Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 45 (2), 372–379. 10.3109/21691401.2016.1160403 - DOI - PubMed
-
- Ben Salem A. N., Zyed R., Lassoued M. A., Nidhal S., Sfar S., Mahjoub A. (2012). Plant-Derived Nanoparticles Enhance Antiviral Activity against Coxsakievirus B3 by Acting on Virus Particles and Vero Cells. Dig. J. Nanomater. Bios. 7 (2), 737–744.
LinkOut - more resources
Full Text Sources

