Long-Term High-Altitude Hypoxia and Alpha Adrenoceptor-Dependent Pulmonary Arterial Contractions in Fetal and Adult Sheep
- PMID: 31555139
- PMCID: PMC6723549
- DOI: 10.3389/fphys.2019.01032
Long-Term High-Altitude Hypoxia and Alpha Adrenoceptor-Dependent Pulmonary Arterial Contractions in Fetal and Adult Sheep
Abstract
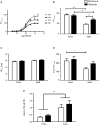
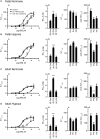
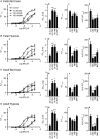
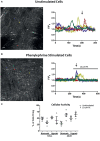
Autonomic innervation of the pulmonary vasculature triggers vasomotor contractility predominately through activation of alpha-adrenergic receptors (α-ARs) in the fetal circulation. Long-term hypoxia (LTH) modulates pulmonary vasoconstriction potentially through upregulation of α1-AR in the vasculature. Our study aimed to elucidate the role of α-AR in phenylephrine (PE)-induced pulmonary vascular contractility, comparing the effects of LTH in the fetal and adult periods on α-AR subtypes and PE-mediated Ca2+ responses and contractions. To address this, we performed wire myography, Ca2+ imaging, and mRNA analysis of pulmonary arteries from ewes and fetuses exposed to LTH or normoxia. Postnatal maturation depressed PE-mediated contractile responses. α2-AR activation contracted fetal vessels; however, this was suppressed by LTH. α1A- and α1B-AR subtypes contributed to arterial contractions in all groups. The α1D-AR was also important to contractility in fetal normoxic vessels and LTH mitigated its function. Postnatal maturity increased the number of myocytes with PE-triggered Ca2+ responses while LTH decreased the percentage of fetal myocytes reacting to PE. The difference between myocyte Ca2+ responsiveness and vessel contractility suggests that fetal arteries are sensitized to changes in Ca2+. The results illustrate that α-adrenergic signaling and vascular function change during development and that LTH modifies adrenergic signaling. These changes may represent components in the etiology of pulmonary vascular disease and foretell the therapeutic potential of adrenergic receptor antagonists in the treatment of pulmonary hypertension.
Keywords: adrenergic receptor; calcium; contraction; fetus; hypoxia; pulmonary artery; sheep.
Figures


References
-
- Barnes P. J., Liu S. F. (1995). Regulation of pulmonary vascular tone. Pharmacol. Rev. 47, 87–131. PMID: - PubMed
-
- Blood A. B., Terry M. H., Merritt T. A., Papamatheakis D. G., Blood Q., Ross J. M., et al. . (2013). Effect of chronic perinatal hypoxia on the role of rho-kinase in pulmonary artery contraction in newborn lambs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R136–R146. 10.1152/ajpregu.00126.2012, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

