Identification of a Novel Functional Corticotropin-Releasing Hormone (CRH2) in Chickens and Its Roles in Stimulating Pituitary TSHβ Expression and ACTH Secretion
- PMID: 31555213
- PMCID: PMC6727040
- DOI: 10.3389/fendo.2019.00595
Identification of a Novel Functional Corticotropin-Releasing Hormone (CRH2) in Chickens and Its Roles in Stimulating Pituitary TSHβ Expression and ACTH Secretion
Abstract
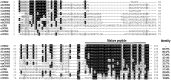
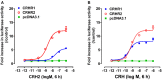
Corticotropin-releasing hormone (CRH), together with its structurally and functionally related neuropeptides, constitute the CRH family and play critical roles in multiple physiological processes. Recently, a novel member of this family, namely CRH2, was identified in vertebrates, however, its functionality and physiological roles remain an open question. In this study, using chicken (c-) as the animal model, we characterized the expression and functionality of CRH2 and investigated its roles in anterior pituitary. Our results showed that (1) cCRH2 cDNA is predicted to encode a 40-aa mature peptide, which shares a higher amino acid sequence identity to cCRH (63%) than to other CRH family peptides (23-38%); (2) Using pGL3-CRE-luciferase reporter system, we demonstrated that cCRH2 is ~15 fold more potent in activating cCRH receptor 2 (CRHR2) than cCRHR1 when expressed in CHO cells, indicating that cCRH2 is bioactive and its action is mainly mediated by CRHR2; (3) Quantitative real-time PCR revealed that cCRH2 is widely expressed in chicken tissues including the hypothalamus and anterior pituitary, and its transcription is likely controlled by promoters near exon 1, which display strong promoter activity in cultured DF-1 and HEK293 cells; (4) In cultured chick pituitary cells, cCRH2 potently stimulates TSHβ expression and shows a lower potency in inducing ACTH secretion, indicating that pituitary/hypothalamic CRH2 can regulate pituitary functions. Collectively, our data provides the first piece of evidence to suggest that CRH2 play roles similar, but non-identical, to those of CRH, such as its differential actions on pituitary, and this helps to elucidate the roles of CRH2 in vertebrates.
Keywords: ACTH and TSH; CRH receptor; CRH2; chicken; pituitary.
Figures


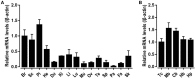



Similar articles
-
Corticotropin-releasing hormone (CRH) stimulates cocaine- and amphetamine-regulated transcript gene (CART1) expression through CRH type 1 receptor (CRHR1) in chicken anterior pituitary.Mol Cell Endocrinol. 2015 Dec 5;417:166-77. doi: 10.1016/j.mce.2015.09.007. Epub 2015 Sep 9. Mol Cell Endocrinol. 2015. PMID: 26363222
-
Characterization of CRH-Binding Protein (CRHBP) in Chickens: Molecular Cloning, Tissue Distribution and Investigation of Its Role as a Negative Feedback Regulator within the Hypothalamus-Pituitary-Adrenal Axis.Genes (Basel). 2022 Sep 20;13(10):1680. doi: 10.3390/genes13101680. Genes (Basel). 2022. PMID: 36292565 Free PMC article.
-
Characterization of a novel thyrotropin-releasing hormone receptor, TRHR3, in chickens.Poult Sci. 2020 Mar;99(3):1643-1654. doi: 10.1016/j.psj.2019.10.062. Epub 2019 Dec 30. Poult Sci. 2020. PMID: 32115036 Free PMC article.
-
Corticotropin-releasing hormone receptor subtypes and emotion.Biol Psychiatry. 1999 Dec 1;46(11):1480-508. doi: 10.1016/s0006-3223(99)00170-5. Biol Psychiatry. 1999. PMID: 10599478 Review.
-
Regulation of pituitary ACTH secretion during chronic stress.Front Neuroendocrinol. 1994 Dec;15(4):321-50. doi: 10.1006/frne.1994.1013. Front Neuroendocrinol. 1994. PMID: 7895891 Review.
Cited by
-
New Insights Into the Evolution of Corticotropin-Releasing Hormone Family With a Special Focus on Teleosts.Front Endocrinol (Lausanne). 2022 Jul 22;13:937218. doi: 10.3389/fendo.2022.937218. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35937826 Free PMC article.
-
KLF7 promotes preadipocyte proliferation via activation of the Akt signaling pathway by Cis-regulating CDKN3.Acta Biochim Biophys Sin (Shanghai). 2022 Oct 25;54(10):1486-1496. doi: 10.3724/abbs.2022144. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36269137 Free PMC article.
-
Interaction between the hypothalamo-pituitary-adrenal and thyroid axes during immobilization stress.Front Physiol. 2022 Oct 18;13:972171. doi: 10.3389/fphys.2022.972171. eCollection 2022. Front Physiol. 2022. PMID: 36330212 Free PMC article.
-
Single-Cell RNA Sequencing Analysis of Chicken Anterior Pituitary: A Bird's-Eye View on Vertebrate Pituitary.Front Physiol. 2021 Jun 29;12:562817. doi: 10.3389/fphys.2021.562817. eCollection 2021. Front Physiol. 2021. PMID: 34267669 Free PMC article.
-
Intrinsically disordered proteins and proteins with intrinsically disordered regions in neurodegenerative diseases.Biophys Rev. 2022 Jun 8;14(3):679-707. doi: 10.1007/s12551-022-00968-0. eCollection 2022 Jun. Biophys Rev. 2022. PMID: 35791387 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

