Synthesis of N-Glycolylneuraminic Acid (Neu5Gc) and Its Glycosides
- PMID: 31555264
- PMCID: PMC6724515
- DOI: 10.3389/fimmu.2019.02004
Synthesis of N-Glycolylneuraminic Acid (Neu5Gc) and Its Glycosides
Abstract
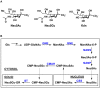
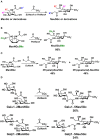
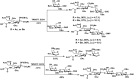
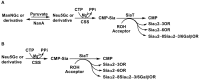
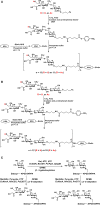
Sialic acids constitute a family of negatively charged structurally diverse monosaccharides that are commonly presented on the termini of glycans in higher animals and some microorganisms. In addition to N-acetylneuraminic acid (Neu5Ac), N-glycolyl neuraminic acid (Neu5Gc) is among the most common sialic acid forms in nature. Nevertheless, unlike most animals, human cells loss the ability to synthesize Neu5Gc although Neu5Gc-containing glycoconjugates have been found on human cancer cells and in various human tissues due to dietary incorporation of Neu5Gc. Some pathogenic bacteria also produce Neu5Ac and the corresponding glycoconjugates but Neu5Gc-producing bacteria have yet to be found. In addition to Neu5Gc, more than 20 Neu5Gc derivatives have been found in non-human vertebrates. To explore the biological roles of Neu5Gc and its naturally occurring derivatives as well as the corresponding glycans and glycoconjugates, various chemical and enzymatic synthetic methods have been developed to obtain a vast array of glycosides containing Neu5Gc and/or its derivatives. Here we provide an overview on various synthetic methods that have been developed. Among these, the application of highly efficient one-pot multienzyme (OPME) sialylation systems in synthesizing compounds containing Neu5Gc and derivatives has been proven as a powerful strategy.
Keywords: Neu5Gc; chemical synthesis; chemoenzymatic synthesis; sialic acid; sialoside.
Figures