TRIM21-From Intracellular Immunity to Therapy
- PMID: 31555278
- PMCID: PMC6722209
- DOI: 10.3389/fimmu.2019.02049
TRIM21-From Intracellular Immunity to Therapy
Abstract
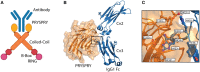
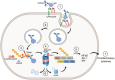
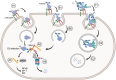
Tripartite motif containing-21 (TRIM21) is a cytosolic ubiquitin ligase and antibody receptor that provides a last line of defense against invading viruses. It does so by acting as a sensor that intercepts antibody-coated viruses that have evaded extracellular neutralization and breached the cell membrane. Upon engagement of the Fc of antibodies bound to viruses, TRIM21 triggers a coordinated effector and signaling response that prevents viral replication while at the same time inducing an anti-viral cellular state. This dual effector function is tightly regulated by auto-ubiquitination and phosphorylation. Therapeutically, TRIM21 has been shown to be detrimental in adenovirus based gene therapy, while it may be favorably utilized to prevent tau aggregation in neurodegenerative disorders. In addition, TRIM21 may synergize with the complement system to block viral replication as well as transgene expression. TRIM21 can also be utilized as a research tool to deplete specific proteins in cells and zebrafish embryos. Here, we review our current biological understanding of TRIM21 in light of its versatile functions.
Keywords: TRIM21; antibody; gene therapy; infection; virus.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

