A Model-System to Address the Impact of Phenotypic Heterogeneity and Plasticity on the Development of Cancer Therapies
- PMID: 31555595
- PMCID: PMC6727362
- DOI: 10.3389/fonc.2019.00842
A Model-System to Address the Impact of Phenotypic Heterogeneity and Plasticity on the Development of Cancer Therapies
Abstract
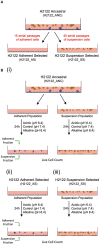
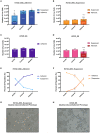
The main challenges in developing effective anti-cancer therapies stem from the highly complex and heterogeneous nature of cancer, including the presence of multiple genetically-encoded and environmentally-induced cancer cell phenotypes within an individual. This diversity can make the development of successful treatments difficult as different phenotypes can have different responses to the same treatment. The lack of model-systems that can be used to simultaneously test the effect of therapies on multiple distinct phenotypic states further contributes to this problem. To mitigate these challenges, we suggest that in vitro model-systems that consist of several genetically-related but phenotypically distinct populations can be used as proxies for the several phenotypes (including adherent and circulating tumor cells) present in a patient with advanced disease. As proof of concept, we have developed such a model and showed that different phenotypes had different responses to the same challenge (i.e., a change in extracellular pH) both in terms of sensitivity and phenotypic plasticity. We suggest that similar model-systems could be developed and used when designing novel therapeutic strategies, to address the potential impact of phenotypic heterogeneity and plasticity of cancer on the development of successful therapies. Specifically, the effect of a therapy should be considered on more than one cancer cell phenotype (to increase its effectiveness), and both cell viability as well as changes in phenotypic state (to address potential plastic responses) should be evaluated. Although we are aware of the limitations of in vitro systems, we believe that the use of established cell lines that express multiple phenotypes can provide invaluable insights into the complex interplay between therapies and cancer's heterogeneous and plastic nature.
Keywords: H2122; experimental evolution; extracellular pH; metastasis; microenvironment; phenotypic plasticity; selection; therapy.
Figures
Similar articles
-
Cancer cells exhibit clonal diversity in phenotypic plasticity.Open Biol. 2017 Feb;7(2):160283. doi: 10.1098/rsob.160283. Open Biol. 2017. PMID: 28202626 Free PMC article.
-
The Intimate Relationship Among EMT, MET and TME: A T(ransdifferentiation) E(nhancing) M(ix) to Be Exploited for Therapeutic Purposes.Cancers (Basel). 2020 Dec 7;12(12):3674. doi: 10.3390/cancers12123674. Cancers (Basel). 2020. PMID: 33297508 Free PMC article. Review.
-
Understanding and leveraging phenotypic plasticity during metastasis formation.NPJ Syst Biol Appl. 2023 Oct 6;9(1):48. doi: 10.1038/s41540-023-00309-1. NPJ Syst Biol Appl. 2023. PMID: 37803056 Free PMC article.
-
Leveraging Cancer Phenotypic Plasticity for Novel Treatment Strategies.J Clin Med. 2024 Jun 5;13(11):3337. doi: 10.3390/jcm13113337. J Clin Med. 2024. PMID: 38893049 Free PMC article.
-
Adaptation to climate change through genetic accommodation and assimilation of plastic phenotypes.Philos Trans R Soc Lond B Biol Sci. 2019 Mar 18;374(1768):20180176. doi: 10.1098/rstb.2018.0176. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30966963 Free PMC article. Review.
Cited by
-
In vitro evidence for the potential of EGFR inhibitors to decrease the TGF-β1-induced dispersal of circulating tumour cell clusters mediated by EGFR overexpression.Sci Rep. 2024 Aug 28;14(1):19980. doi: 10.1038/s41598-024-70358-x. Sci Rep. 2024. PMID: 39198539 Free PMC article.
-
Synergistic inter-clonal cooperation involving crosstalk, co-option and co-dependency can enhance the invasiveness of genetically distant cancer clones.BMC Ecol Evol. 2023 May 24;23(1):20. doi: 10.1186/s12862-023-02129-7. BMC Ecol Evol. 2023. PMID: 37226092 Free PMC article.
-
Agent-based modelling reveals strategies to reduce the fitness and metastatic potential of circulating tumour cell clusters.Evol Appl. 2020 Mar 18;13(7):1635-1650. doi: 10.1111/eva.12943. eCollection 2020 Aug. Evol Appl. 2020. PMID: 32821275 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

