Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms
- PMID: 31555796
- PMCID: PMC7080342
- DOI: 10.1210/endrev/bnz004
Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms
Abstract
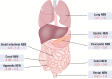
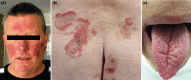
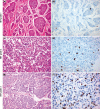
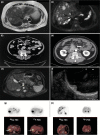
Neuroendocrine neoplasms constitute a diverse group of tumors that derive from the sensory and secretory neuroendocrine cells and predominantly arise within the pulmonary and gastrointestinal tracts. The majority of these neoplasms have a well-differentiated grade and are termed neuroendocrine tumors (NETs). This subgroup is characterized by limited proliferation and patients affected by these tumors carry a good to moderate prognosis. A substantial subset of patients presenting with a NET suffer from the consequences of endocrine syndromes as a result of the excessive secretion of amines or peptide hormones, which can impair their quality of life and prognosis. Over the past 15 years, critical developments in tumor grading, diagnostic biomarkers, radionuclide imaging, randomized controlled drug trials, evidence-based guidelines, and superior prognostic outcomes have substantially altered the field of NET care. Here, we review the relevant advances to clinical practice that have significantly upgraded our approach to NET patients, both in diagnostic and in therapeutic options.
© Endocrine Society 2020.
Figures

References
-
- Perren A, Couvelard A, Scoazec JY, et al. ; Antibes Consensus Conference participants . ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pathology: diagnosis and prognostic stratification. Neuroendocrinology. 2017;105(3):196–200. - PubMed
-
- Inzani F, Petrone G, Rindi G. The New World Health Organization classification for pancreatic neuroendocrine neoplasia. Endocrinol Metab Clin North Am. 2018;47(3):463–470. - PubMed
-
- Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25(3):458–511. - PubMed
-
- Asa SL, Casar-Borota O, Chanson P, et al. ; attendees of 14th Meeting of the International Pituitary Pathology Club, Annecy, France, November 2016. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an International Pituitary Pathology Club proposal. Endocr Relat Cancer. 2017;24(4):C5–C8. - PubMed
-
- Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

