Safety, Tolerability, and Immunogenicity of Plasmodium falciparum Sporozoite Vaccine Administered by Direct Venous Inoculation to Infants and Young Children: Findings From an Age De-escalation, Dose-Escalation, Double-blind, Randomized Controlled Study in Western Kenya
- PMID: 31555824
- PMCID: PMC7428396
- DOI: 10.1093/cid/ciz925
Safety, Tolerability, and Immunogenicity of Plasmodium falciparum Sporozoite Vaccine Administered by Direct Venous Inoculation to Infants and Young Children: Findings From an Age De-escalation, Dose-Escalation, Double-blind, Randomized Controlled Study in Western Kenya
Abstract
Background: The whole Plasmodium falciparum sporozoite (PfSPZ) vaccine is being evaluated for malaria prevention. The vaccine is administered intravenously for maximal efficacy. Direct venous inoculation (DVI) with PfSPZ vaccine has been safe, tolerable, and feasible in adults, but safety data for children and infants are limited.
Methods: We conducted an age de-escalation, dose-escalation randomized controlled trial in Siaya County, western Kenya. Children and infants (aged 5-9 years, 13-59 months, and 5-12 months) were enrolled into 13 age-dose cohorts of 12 participants and randomized 2:1 to vaccine or normal saline placebo in escalating doses: 1.35 × 105, 2.7 × 105, 4.5 × 105, 9.0 × 105, and 1.8 × 106 PfSPZ, with the 2 highest doses given twice, 8 weeks apart. Solicited adverse events (AEs) were monitored for 8 days after vaccination, unsolicited AEs for 29 days, and serious AEs throughout the study. Blood taken prevaccination and 1 week postvaccination was tested for immunoglobulin G antibodies to P. falciparum circumsporozoite protein (PfCSP) using enzyme-linked immunosorbent assay.
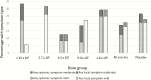
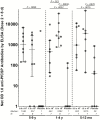
Results: Rates of AEs were similar in vaccinees and controls for solicited (35.7% vs 41.5%) and unsolicited (83.9% vs 92.5%) AEs, respectively. No related grade 3 AEs, serious AEs, or grade 3 laboratory abnormalities occurred. Most (79.0%) vaccinations were administered by a single DVI. Among those in the 9.0 × 105 and 1.8 × 106 PfSPZ groups, 36 of 45 (80.0%) vaccinees and 4 of 21 (19.0%) placebo controls developed antibodies to PfCSP (P < .001).
Conclusions: PfSPZ vaccine in doses as high as 1.8 × 106 can be administered to infants and children by DVI, and was safe, well tolerated, and immunogenic.
Clinical trials registration: NCT02687373.
Keywords: infants; malaria; safety; sporozoite; vaccine.
Published by Oxford University Press for the Infectious Diseases Society of America 2019. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures


References
-
- World Health Organization. World malaria report 2017. Geneva, Switzerland: WHO, 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

