Complement in malaria immunity and vaccines
- PMID: 31556468
- PMCID: PMC6972673
- DOI: 10.1111/imr.12802
Complement in malaria immunity and vaccines
Abstract
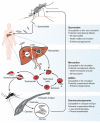
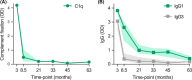
Developing efficacious vaccines for human malaria caused by Plasmodium falciparum is a major global health priority, although this has proven to be immensely challenging over the decades. One major hindrance is the incomplete understanding of specific immune responses that confer protection against disease and/or infection. While antibodies to play a crucial role in malaria immunity, the functional mechanisms of these antibodies remain unclear as most research has primarily focused on the direct inhibitory or neutralizing activity of antibodies. Recently, there is a growing body of evidence that antibodies can also mediate effector functions through activating the complement system against multiple developmental stages of the parasite life cycle. These antibody-complement interactions can have detrimental consequences to parasite function and viability, and have been significantly associated with protection against clinical malaria in naturally acquired immunity, and emerging findings suggest these mechanisms could contribute to vaccine-induced immunity. In order to develop highly efficacious vaccines, strategies are needed that prioritize the induction of antibodies with enhanced functional activity, including the ability to activate complement. Here we review the role of complement in acquired immunity to malaria, and provide insights into how this knowledge could be used to harness complement in malaria vaccine development.
Keywords: antibodies; complement; immunity; malaria; vaccines.
© 2019 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- World Health Organization . World Malaria Report 2018. Geneva: World Health Organisation; 2018.
-
- Beeson JG, Kurtovic L, Dobaño C, et al. Challenges and strategies for developing efficacious and long‐lasting malaria vaccines. Sci Transl Med. 2019;11:eaau1458. - PubMed
-
- Cohen S, McGregor IA, Carrington S. Gamma‐globulin and acquired immunity to human malaria. Nature. 1961;192:733‐737. - PubMed
-
- Richards JS, Beeson JG. The future for blood‐stage vaccines against malaria. Immunol Cell Biol. 2009;87:377‐390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

