Factors Associated With Successful Discontinuation of Immune Suppression After Allogeneic Hematopoietic Cell Transplantation
- PMID: 31556923
- PMCID: PMC6763979
- DOI: 10.1001/jamaoncol.2019.2974
Factors Associated With Successful Discontinuation of Immune Suppression After Allogeneic Hematopoietic Cell Transplantation
Abstract
Importance: Immune suppression discontinuation is routinely attempted after allogeneic hematopoietic cell transplantation (HCT) and under current practices may lead to graft-vs-host disease (GVHD)-associated morbidity and death. However, the likelihood and predictive factors associated with successful immune suppression discontinuation after HCT are poorly understood.
Objectives: To examine factors associated with successful immune suppression discontinuation and risk for immune suppression discontinuation failure under conventional HCT approaches and develop a practical tool to estimate successful immune suppression discontinuation likelihood at the clinical point of care.
Design, setting, and participants: Using long-term follow-up data from 2 national Blood and Marrow Transplant Clinical Trial Network studies (N = 827), a multistate model was developed to investigate the probability and variables associated with immune suppression discontinuation success. The study began in July 2015, and analyses were completed in August 2019.
Main outcomes and measures: Immune suppression discontinuation and immune suppression discontinuation failure.
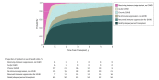
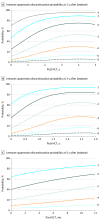
Results: Of the 827 patients included in the analysis, 456 were men (55.1%). Median age at transplant was 44 (range, <1-67) years. With median follow-up of 72 (range, 11-124) months, 20.0% of the patients were alive and not receiving immune suppression at 5 years. Older recipient age (adjusted odds ratio [aOR] of >50 vs <30 years, 0.27, 99% CI, 0.14-0.50; P < .001), mismatched unrelated donor (aOR, mismatched unrelated vs matched related, 0.37; 99% CI, 0.14-0.97; P = .008), peripheral blood graft (aOR of peripheral blood graft vs bone marrow, 0.46; 99% CI, 0.26-0.82; P < .001), and advanced stage disease (aOR of advanced vs early disease, 0.45; 99% CI, 0.23-0.86, P = 0.002), were significantly associated with decreased odds of immune suppression discontinuation. Failed attempts at immune suppression discontinuation (127 patients [37.1% of total immune suppression discontinuation events]) resulting in GVHD were significantly associated with use of peripheral blood stem cells (HR, 2.62; 99% CI, 1.30-5.29; P < .001), prior GVHD, and earlier immune suppression discontinuation attempts. Earlier immune suppression discontinuation was not associated with protection from cancer relapse after HCT (adjusted hazard ratio for discontinuation vs not, 1.95; 99% CI, 0.88-4.31; P = .03).Dynamic prediction models were developed to provide future immune suppression discontinuation probability according to individual patient characteristics.
Conclusions and relevance: Successful immune suppression discontinuation is uncommon in the setting of peripheral blood stem cell grafts. The data suggest earlier attempts at ISD conferred no long-term benefit, given frequent ISD failure, limited subsequent success after initial failed ISD attempt, and no evidence of relapse reduction. Using a risk model-based clinical application, physicians may be able to identify individual patients' probability of successful immune suppression discontinuation.
Conflict of interest statement
Figures

References
-
- Mengarelli A, Iori AP, Romano A, et al. One-year cyclosporine prophylaxis reduces the risk of developing extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Haematologica. 2003;88(3):315-323. - PubMed

