Systems analysis-based assessment of post-treatment adverse events in lymphatic filariasis
- PMID: 31557154
- PMCID: PMC6762072
- DOI: 10.1371/journal.pntd.0007697
Systems analysis-based assessment of post-treatment adverse events in lymphatic filariasis
Abstract
Background: Lymphatic filariasis (LF) is a neglected tropical disease, and the Global Program to Eliminate LF delivers mass drug administration (MDA) to 500 million people every year. Adverse events (AEs) are common after LF treatment.
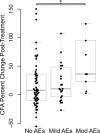
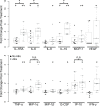
Methodology/principal findings: To better understand the pathogenesis of AEs, we studied LF-patients from a treatment trial. Plasma levels of many filarial antigens increased post-treatment in individuals with AEs, and this is consistent with parasite death. Circulating immune complexes were not elevated in these participants, and the classical complement cascade was not activated. Multiple cytokines increased after treatment in persons with AEs. A transcriptomic analysis was performed for nine individuals with moderate systemic AEs and nine matched controls. Differential gene expression analysis identified a significant transcriptional signature associated with post-treatment AEs; 744 genes were upregulated. The transcriptional signature was enriched for TLR and NF-κB signaling. Increased expression of seven out of the top eight genes upregulated in persons with AEs were validated by qRT-PCR, including TLR2.
Conclusions/significance: This is the first global study of changes in gene expression associated with AEs after treatment of lymphatic filariasis. Changes in cytokines were consistent with prior studies and with the RNAseq data. These results suggest that Wolbachia lipoprotein is involved in AE development, because it activates TLR2-TLR6 and downstream NF-κB. Additionally, LPS Binding Protein (LBP, which shuttles lipoproteins to TLR2) increased post-treatment in individuals with AEs. Improved understanding of the pathogenesis of AEs may lead to improved management, increased MDA compliance, and accelerated LF elimination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


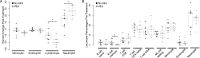

References
-
- Simonsen PE, Fischer PU, Hoerauf A, Weil GJ. The Filariases In: Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson's Tropical Diseases. 23rd ed: Elsevier; 2013. p. 737–65.
-
- WHO. Global programme to eliminate lymphatic filariasis: progress report, 2016. Global programme to eliminate lymphatic filariasis: progress report, 2016. 2017 2 October 2017; (No. 40, 2017, 91, 589–608):[20 p. p.]. Available from: https://www.who.int/lymphatic_filariasis/resources/who_wer9240/en/.
-
- Ouattara AF KO, Bjerum C, Koudou BG, Meite A, Kazura JW, Weil G and King CL. High efficacy of singe dose of co-administered ivermecting, diethylcarbamazine and albendazole in treatment of lymphatic filariasis in Cote d'Ivoire. American Society of Tropical Medicine and Hygiene. 2016; 95(5):[600 p.]. Available from: https://www.astmh.org/ASTMH/media/Documents/ASTMH-2016-Annual-Meeting-Ab....
-
- Bjerum CM OA, Koudou BG, Meite A, Kazura JW, Weil G and King CL. The macrofilaricidal activity of a single dose of ivermectin, albendazole and diethylcarbamazine against Wuchereria bancrofti in Cote d'Ivoire. American Society of Tropical Medicine and Hygiene. 2016; 95(5):[599 p.]. Available from: https://www.astmh.org/ASTMH/media/Documents/ASTMH-2016-Annual-Meeting-Ab....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

