Multi-omics analysis identifies mitochondrial pathways associated with anxiety-related behavior
- PMID: 31557158
- PMCID: PMC6762065
- DOI: 10.1371/journal.pgen.1008358
Multi-omics analysis identifies mitochondrial pathways associated with anxiety-related behavior
Abstract
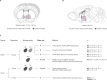
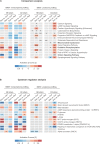
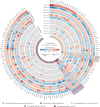
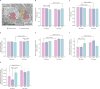
Stressful life events are major environmental risk factors for anxiety disorders, although not all individuals exposed to stress develop clinical anxiety. The molecular mechanisms underlying the influence of environmental effects on anxiety are largely unknown. To identify biological pathways mediating stress-related anxiety and resilience to it, we used the chronic social defeat stress (CSDS) paradigm in male mice of two inbred strains, C57BL/6NCrl (B6) and DBA/2NCrl (D2), that differ in their susceptibility to stress. Using a multi-omics approach, we identified differential mRNA, miRNA and protein expression changes in the bed nucleus of the stria terminalis (BNST) and blood cells after chronic stress. Integrative gene set enrichment analysis revealed enrichment of mitochondrial-related genes in the BNST and blood of stressed mice. To translate these results to human anxiety, we investigated blood gene expression changes associated with exposure-induced panic attacks. Remarkably, we found reduced expression of mitochondrial-related genes in D2 stress-susceptible mice and in exposure-induced panic attacks in humans, but increased expression of these genes in B6 stress-susceptible mice. Moreover, stress-susceptible vs. stress-resilient B6 mice displayed more mitochondrial cross-sections in the post-synaptic compartment after CSDS. Our findings demonstrate mitochondrial-related alterations in gene expression as an evolutionarily conserved response in stress-related behaviors and validate the use of cross-species approaches in investigating the biological mechanisms underlying anxiety disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

