Does Mindfulness-Based Cognitive Therapy for Migraine Reduce Migraine-Related Disability in People with Episodic and Chronic Migraine? A Phase 2b Pilot Randomized Clinical Trial
- PMID: 31557329
- PMCID: PMC6788949
- DOI: 10.1111/head.13657
Does Mindfulness-Based Cognitive Therapy for Migraine Reduce Migraine-Related Disability in People with Episodic and Chronic Migraine? A Phase 2b Pilot Randomized Clinical Trial
Abstract
Objective: The current Phase 2b study aimed to evaluate the efficacy of mindfulness-based cognitive therapy for migraine (MBCT-M) to reduce migraine-related disability in people with migraine.
Background: Mindfulness-based interventions represent a promising avenue to investigate effects in people with migraine. MBCT teaches mindfulness meditation and cognitive-behavioral skills and directly applies these skills to address disease-related cognitions.
Methods: Participants with migraine (6-30 headache days/month) were recruited from neurology office referrals and local and online advertisements in the broader New York City area. During the 30-day baseline period, all participants completed a daily headache diary. Participants who met inclusion and exclusion criteria were randomized in a parallel design, stratified by chronic migraine status, to receive either 8 weekly individual MBCT-M sessions or 8 weeks of waitlist/treatment as usual (WL/TAU). All participants completed surveys including primary outcome evaluations at Months 0, 1, 2, and 4. All participants completed a headache diary during the 30-day posttreatment evaluation period. Primary outcomes were the change from Month 0 to Month 4 in the headache disability inventory (HDI) and the Migraine Disability Assessment (MIDAS) (total score ≥ 21 indicating severe disability); secondary outcomes (headache days/30 days, average headache attack pain intensity, and attack-level migraine-related disability [Migraine Disability Index (MIDI)]) were derived from the daily headache diary.
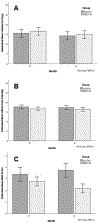
Results: Sixty participants were randomized to receive MBCT-M (n = 31) or WL/TAU (n = 29). Participants (M age = 40.1, SD = 11.7) were predominantly White (n = 49/60; 81.7%) and Non-Hispanic (N = 50/60; 83.3%) women (n = 55/60; 91.7%) with a graduate degree (n = 35/60; 55.0%) who were working full-time (n = 38/60; 63.3%). At baseline, the average HDI score (51.4, SD = 19.0) indicated a moderate level of disability and the majority of participants (50/60, 83.3%) fell in the "Severe Disability" range in the MIDAS. Participants recorded an average of 16.0 (SD = 5.9) headache days/30 days, with an average headache attack pain intensity of 1.7 on a 4-point scale (SD = 0.3), indicating moderate intensity. Average levels of daily disability reported on the MIDI were 3.1/10 (SD = 1.8). For the HDI, mean scores decreased more from Month 0 to Month 4 in the MBCT-M group (-14.3) than the waitlist/treatment as an usual group (-0.2; P < .001). For the MIDAS, the group*month interaction was not significant when accounting for the divided alpha, P = .027; across all participants in both groups, the estimated proportion of participants falling in the "Severe Disability" category fell significantly from 88.3% at Month 0 to 66.7% at Month 4, P < .001. For diary-reported headache days/30 days an average headache attack pain intensity, neither the group*month interaction (Ps = .773 and .888, respectively) nor the time effect (Ps = .059 and .428, respectively) was significant. Mean MIDI scores decreased in the MBCT-M group (-0.6/10), whereas they increased in the waitlist/treatment as an usual group (+0.3/10), P = .007.
Conclusions: MBCT-M demonstrated efficacy to reduce headache-related disability and attack-level migraine-related disability. MBCT-M is a promising emerging treatment for addressing migraine-related disability.
Keywords: behavioral treatment; disability; migraine; mindfulness; therapy.
© 2019 American Headache Society.
Conflict of interest statement
Conflict of Interest:
Dr. Seng receives research support from the NINDS (K23 NS096107 PI: Seng) and has consulted for GlaxoSmithKline, Eli Lilly, and received honoraria from Haymarket Media. Dr. Seng has received travel funds from the American Psychological Association. Dr. Metts is the developer of the status/post mobile application used in this study. Development was conducted in his role as President of Infinite Arms, LLC, which maintains ownership of the Intellectual Property associated with this application. Dr. Minen has received grant support from the NIH, the American Academy of Neurology, the American Brain Foundation, the National Multiple Sclerosis Society and from internal grants from NYU. She has also received honorarium from the American Academy of Neurology, the National Headache Foundation, and Barnard College. Dr. Minen has received travel funds to attend meetings from the American Headache Society and the American Academy of Neurology. Dr. Minen serves as Co-Section Head of the Headache Section of Pain Medicine as an Associate Editor of the journal Headache. Dr. Buse receives grant support and honoraria from Allergan, Amgen/Novartis, Avanir, Biohaven, Dr. Reddy’s Laboratories/Promius Pharma, Eli Lilly and Teva and for serving on the editorial board of Current Pain and Headache Reports. Dr. Lipton receives grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund and serves as consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol Myers Squibb, Cognimed, CoLucid, Dr. Reddy’s Laboratories/Promius, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (Oxford University Press, 2009). Drs. Singer, Grinberg and Minen, and Ms. Patel and Rosenberg, report no disclosures.
Figures


References
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition Cephalalgia: 2018;38:1–211. - PubMed
-
- Lipton R, Bigal M, Diamond M, Freitag F, Reed M, Stewart W. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. - PubMed
-
- Buse D, Manack A, Serrano D, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache. 2012;52:3–17. - PubMed
-
- Lipton R, Stewart A, Diamond S, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

