A Long-Lived Azafullerenyl Radical Stabilized by Supramolecular Shielding with a [10]Cycloparaphenylene
- PMID: 31557367
- PMCID: PMC7003913
- DOI: 10.1002/anie.201909126
A Long-Lived Azafullerenyl Radical Stabilized by Supramolecular Shielding with a [10]Cycloparaphenylene
Abstract
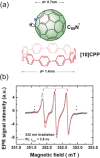
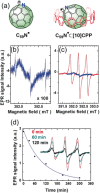
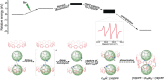
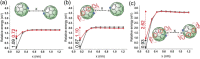
A major handicap towards the exploitation of radicals is their inherent instability. In the paramagnetic azafullerenyl radical C59 N. , the unpaired electron is strongly localized next to the nitrogen atom, which induces dimerization to diamagnetic bis(azafullerene), (C59 N)2 . Conventional stabilization by introducing steric hindrance around the radical is inapplicable here because of the concave fullerene geometry. Instead, we developed an innovative radical shielding approach based on supramolecular complexation, exploiting the protection offered by a [10]cycloparaphenylene ([10]CPP) nanobelt encircling the C59 N. radical. Photoinduced radical generation is increased by a factor of 300. The EPR signal showing characteristic 14 N hyperfine splitting of C59 N. ⊂ [10]CPP was traced even after several weeks, which corresponds to a lifetime increase of >108 . The proposed approach can be generalized by tuning the diameter of the employed nanobelts, opening new avenues for the design and exploitation of radical fullerenes.
Keywords: [10]cycloparaphenylene; azafullerenes; host-guest complexes; long-lived radicals; photoinduced radical generation.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Umeyama T., Imahori H., Nanoscale Horiz. 2018, 3, 352–366. - PubMed
-
- Kirner S., Sekita M., Guldi D. M., Adv. Mater. 2014, 26, 1482–1493. - PubMed
-
- Casu M. B., Acc. Chem. Res. 2018, 51, 753–760. - PubMed
-
- Ardavan A., Blundell S. J., J. Mater. Chem. 2009, 19, 1754–1760.
-
- Benjamin S. C., Ardavan A., Briggs G. A. D., Britz D. A., Gunlycke D., Jefferson J., Jones M. A. G., Leigh D. F., Lovett B. W., Khlobystov A. N., Lyon S. A., Morton J. J. L., Porfyrakis K., Sambrook M. R., Tyryshkin A. M., J. Phys. Condens. Matter 2006, 18, S867–S883.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

