Preclinical evaluation of exemestane as a novel chemotherapy for gastric cancer
- PMID: 31557413
- PMCID: PMC6815818
- DOI: 10.1111/jcmm.14605
Preclinical evaluation of exemestane as a novel chemotherapy for gastric cancer
Abstract
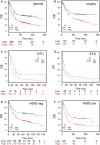
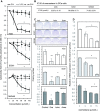
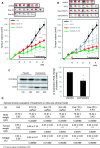
CYP19A1/aromatase (Ar) is a prognostic biomarker of gastric cancer (GCa). Ar is a critical enzyme for converting androstenedione to oestradiol in the steroidogenesis cascade. For decades, Ar has been targeted with Ar inhibitors (ARIs) in gynaecologic malignancies; however, it is unexplored in GCa. A single-cohort tissue microarray examination was conducted to study the association between Ar expression and disease outcome in Asian patients with GCa. The results revealed that Ar was a prognostic promoter. Bioinformatics analyses conducted on a Caucasian-based cDNA microarray databank showed Ar to be positively associated with GCa prognosis for multiple clinical modalities, including surgery, 5-Fluorouracil (5-FU) for adjuvant chemotherapy, or HER2 positivity. These findings imply that targeting Ar expression exhibits a potential for fulfilling unmet medical needs. Hence, Ar-targeting compounds were tested, and the results showed that exemestane exhibited superior cancer-suppressing efficacy to other ARIs. In addition, exemestane down-regulated Ar expression. Ablating Ar abundance with short hairpin (sh)Ar could also suppress GCa cell growth, and adding 5-FU could facilitate this effect. Notably, adding oestradiol could not prevent exemestane or shAr effects, implicating a nonenzymatic mechanism of Ar in cancer growth. Regarding translational research, treatment with exemestane alone exhibited tumour suppression efficacy in a dose-dependent manner. Combining subminimal doses of 5-FU and exemestane exerted an excellent tumour suppression effect without influencing bodyweight. This study validated the therapeutic potentials of exemestane in GCa. Combination of metronomic 5-FU and exemestane for GCa therapy is recommended.
Keywords: aromatase; exemestane; gastric cancer.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
There is no conflict of interests in this work.
Figures


Similar articles
-
Ursolic acid silences CYP19A1/aromatase to suppress gastric cancer growth.Cancer Med. 2022 Jul;11(14):2824-2835. doi: 10.1002/cam4.4536. Epub 2022 May 11. Cancer Med. 2022. PMID: 35545835 Free PMC article.
-
Cholesterol import and steroidogenesis are biosignatures for gastric cancer patient survival.Oncotarget. 2017 Jan 3;8(1):692-704. doi: 10.18632/oncotarget.13524. Oncotarget. 2017. PMID: 27893427 Free PMC article.
-
Role of obesity on the efficacy of exemestane plus ovarian suppression in hormone receptor-positive premenopausal breast cancer.Future Oncol. 2015;11(6):905-7. doi: 10.2217/fon.15.6. Future Oncol. 2015. PMID: 25760972 No abstract available.
-
Exemestane: a novel aromatase inactivator for breast cancer.Clin Breast Cancer. 2000 Oct;1(3):211-6. doi: 10.3816/CBC.2000.n.017. Clin Breast Cancer. 2000. PMID: 11899645 Review.
-
Experience with exemestane in the treatment of early and advanced breast cancer.Expert Opin Drug Metab Toxicol. 2008 Jul;4(7):987-97. doi: 10.1517/17425255.4.7.987. Expert Opin Drug Metab Toxicol. 2008. PMID: 18624685 Review.
Cited by
-
Metronomic chemotherapy in cancer treatment: new wine in an old bottle.Theranostics. 2024 Jun 1;14(9):3548-3564. doi: 10.7150/thno.95619. eCollection 2024. Theranostics. 2024. PMID: 38948068 Free PMC article. Review.
-
Identification of the NRF2 transcriptional network as a therapeutic target for trigeminal neuropathic pain.Sci Adv. 2022 Aug 5;8(31):eabo5633. doi: 10.1126/sciadv.abo5633. Epub 2022 Aug 3. Sci Adv. 2022. PMID: 35921423 Free PMC article.
-
Targeting inhibition of prognosis-related lipid metabolism genes including CYP19A1 enhances immunotherapeutic response in colon cancer.J Exp Clin Cancer Res. 2023 Apr 13;42(1):85. doi: 10.1186/s13046-023-02647-8. J Exp Clin Cancer Res. 2023. PMID: 37055842 Free PMC article.
-
Inflammation-Related Genes Serve as Prognostic Biomarkers and Involve in Immunosuppressive Microenvironment to Promote Gastric Cancer Progression.Front Med (Lausanne). 2022 Mar 16;9:801647. doi: 10.3389/fmed.2022.801647. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35372408 Free PMC article.
-
The Role of Lipid Metabolism in Gastric Cancer.Front Oncol. 2022 Jun 15;12:916661. doi: 10.3389/fonc.2022.916661. eCollection 2022. Front Oncol. 2022. PMID: 35785165 Free PMC article. Review.
References
-
- Gonzalez CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter. 2013;18(Suppl 1):34‐38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

