Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren's Syndrome-Like Disease
- PMID: 31557796
- PMCID: PMC6801785
- DOI: 10.3390/ijms20194750
Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren's Syndrome-Like Disease
Erratum in
-
Correction: Abughanam, G., et al. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren's Syndrome-Like Disease. Int. J. Mol. Sci. 2019, 20, 4750.Int J Mol Sci. 2021 Jan 18;22(2):894. doi: 10.3390/ijms22020894. Int J Mol Sci. 2021. PMID: 33477738 Free PMC article.
Abstract
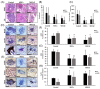
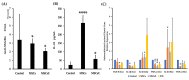
Sjogren's syndrome (SS) is an autoimmune disease that manifests primarily in salivary and lacrimal glands leading to dry mouth and eyes. Unfortunately, there is no cure for SS due to its complex etiopathogenesis. Mesenchymal stem cells (MSCs) were successfully tested for SS, but some risks and limitations remained for their clinical use. This study combined cell- and biologic-based therapies by utilizing the MSCs extract (MSCsE) to treat SS-like disease in NOD mice. We found that MSCsE and MSCs therapies were successful and comparable in preserving salivary and lacrimal glands function in NOD mice when compared to control group. Cells positive for AQP5, AQP4, α-SMA, CK5, and c-Kit were preserved. Gene expression of AQP5, EGF, FGF2, BMP7, LYZ1 and IL-10 were upregulated, and downregulated for TNF-α, TGF-β1, MMP2, CASP3, and IL-1β. The proliferation rate of the glands and serum levels of EGF were also higher. Cornea integrity and epithelial thickness were maintained due to tear flow rate preservation. Peripheral tolerance was re-established, as indicated by lower lymphocytic infiltration and anti-SS-A antibodies, less BAFF secretion, higher serum IL-10 levels and FoxP3+ Treg cells, and selective inhibition of B220+ B cells. These promising results opened new venues for a safer and more convenient combined biologic- and cell-based therapy.
Keywords: Sjogren’s syndrome (ss); autoimmune diseases; biologic therapy; bone marrow; cell extract; lacrimal gland; mesenchymal stem cells (MSCs); non-obese diabetic mice (NOD); salivary glands; submandibular glands.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

