The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers
- PMID: 31557908
- PMCID: PMC6826960
- DOI: 10.3390/cancers11101429
The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers
Abstract
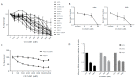
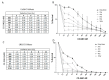
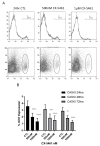
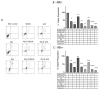
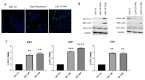
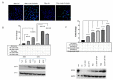
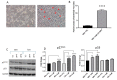
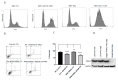
An increased rate of cellular proliferation is a hallmark of cancer and may be accompanied by an increase in ribosome biogenesis and dysregulation in rRNA synthesis. In this regard, CX-5461 has been developed as a novel RNA polymerase I inhibitor and is currently in Phase I/II clinical trials for solid and hematological malignancies. In the present study, interactions between CX-5461 and single-dose X-ray exposure were assessed using isobologram analysis using MTS assay and drug-induced cell death was assessed using flow cytometric, confocal microscopy and Western blot analysis. Combination treatments involving CX-5461 and single-dose X-ray exposure highlighted increased effectiveness compared to individual treatment alone in the CaSki cervical cancer line, with marked synergistic interaction occurring within the low-drug (50 nM) and low-dose radiation range (2-6 Gy). Cell lines challenged with CX-5461 demonstrated the presence of DNA damage, induction of apoptosis, autophagy and senescence alongside high percentages of G2/M cell cycle arrest. In addition, we report preferential sensitivity of ovarian cancer cells with BRCA2 mutation to this novel agent. Taken together, CX-5461 displayed a broad spectrum of activity in a panel of solid cancer cell lines with IC50 values ranging from 35 nM to >1 µM. The work described herein identifies the synergistic effects of CX-5461 in combination with X-rays in solid cancers and may also aid in the design of clinical trials involving this novel agent.
Keywords: CX-5461; RNA polymerase I targeting; combination studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CX-5461 induces radiosensitization through modification of the DNA damage response and not inhibition of RNA polymerase I.Sci Rep. 2022 Mar 8;12(1):4059. doi: 10.1038/s41598-022-07928-4. Sci Rep. 2022. PMID: 35260696 Free PMC article.
-
rRNA synthesis inhibitor, CX-5461, activates ATM/ATR pathway in acute lymphoblastic leukemia, arrests cells in G2 phase and induces apoptosis.Oncotarget. 2015 Jul 20;6(20):18094-104. doi: 10.18632/oncotarget.4093. Oncotarget. 2015. PMID: 26061708 Free PMC article.
-
Transient rRNA synthesis inhibition with CX-5461 is sufficient to elicit growth arrest and cell death in acute lymphoblastic leukemia cells.Oncotarget. 2015 Oct 27;6(33):34846-58. doi: 10.18632/oncotarget.5413. Oncotarget. 2015. PMID: 26472108 Free PMC article.
-
Targeting RNA-Polymerase I in Both Chemosensitive and Chemoresistant Populations in Epithelial Ovarian Cancer.Clin Cancer Res. 2017 Nov 1;23(21):6529-6540. doi: 10.1158/1078-0432.CCR-17-0282. Epub 2017 Aug 4. Clin Cancer Res. 2017. PMID: 28778862 Free PMC article.
-
The Potential of Targeting Ribosome Biogenesis in High-Grade Serous Ovarian Cancer.Int J Mol Sci. 2017 Jan 20;18(1):210. doi: 10.3390/ijms18010210. Int J Mol Sci. 2017. PMID: 28117679 Free PMC article. Review.
Cited by
-
Quinacrine Induces Nucleolar Stress in Treatment-Refractory Ovarian Cancer Cell Lines.Cancers (Basel). 2021 Sep 16;13(18):4645. doi: 10.3390/cancers13184645. Cancers (Basel). 2021. PMID: 34572872 Free PMC article.
-
The chemotherapeutic agent CX-5461 irreversibly blocks RNA polymerase I initiation and promoter release to cause nucleolar disruption, DNA damage and cell inviability.NAR Cancer. 2020 Dec;2(4):zcaa032. doi: 10.1093/narcan/zcaa032. Epub 2020 Nov 6. NAR Cancer. 2020. PMID: 33196044 Free PMC article.
-
DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer.Signal Transduct Target Ther. 2020 May 1;5(1):60. doi: 10.1038/s41392-020-0150-x. Signal Transduct Target Ther. 2020. PMID: 32355263 Free PMC article. Review.
-
Targeting Protein Synthesis in Colorectal Cancer.Cancers (Basel). 2020 May 21;12(5):1298. doi: 10.3390/cancers12051298. Cancers (Basel). 2020. PMID: 32455578 Free PMC article. Review.
-
Targeting autophagy promotes the antitumor effect of radiotherapy on cervical cancer cells.Cancer Biol Ther. 2024 Dec 31;25(1):2431136. doi: 10.1080/15384047.2024.2431136. Epub 2024 Dec 5. Cancer Biol Ther. 2024. PMID: 39635971 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

