Taking up Cancer Immunotherapy Challenges: Bispecific Antibodies, the Path Forward?
- PMID: 31557983
- PMCID: PMC6698871
- DOI: 10.3390/antib5010001
Taking up Cancer Immunotherapy Challenges: Bispecific Antibodies, the Path Forward?
Abstract
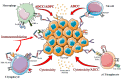
As evidenced by the recent approvals of Removab (EU, Trion Pharma) in 2009 and of Blincyto (US, Amgen) in 2014, the high potential of bispecific antibodies in the field of immuno-oncology is eliciting a renewed interest from pharmaceutical companies. Supported by rapid advances in antibody engineering and the development of several technological platforms such as Triomab or bispecific T cell engagers (BiTEs), the "bispecifics" market has increased significantly over the past decade and may occupy a pivotal space in the future. Over 30 bispecific molecules are currently in different stages of clinical trials and more than 70 in preclinical phase. This review focuses on the clinical potential of bispecific antibodies as immune effector cell engagers in the onco-immunotherapy field. We summarize current strategies targeting various immune cells and their clinical interests. Furthermore, perspectives of bispecific antibodies in future clinical developments are addressed.
Keywords: NK cells; T-cells; bispecific antibody; cancer immunotherapy; immune effector cells; immuno-checkpoint.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thomas L. Discussion. In: Lawrence H.S., editor. Cellular and Humoral Aspects of the Hypersensitive States. Hoeber-Harper; New York, NY, USA: 1959. pp. 529–533.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

