Physician characteristics associated with treatment initiation patterns in idiopathic pulmonary fibrosis
- PMID: 31558049
- PMCID: PMC6764076
- DOI: 10.1177/1479973119879678
Physician characteristics associated with treatment initiation patterns in idiopathic pulmonary fibrosis
Abstract
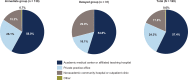
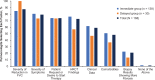
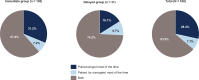
Pirfenidone and nintedanib are oral antifibrotic agents approved for the treatment of idiopathic pulmonary fibrosis (IPF). Real-world data on factors that influence IPF treatment decisions are limited. Physician characteristics associated with antifibrotic therapy initiation following an IPF diagnosis were examined in a sample of US pulmonologists. An online, self-administered survey was fielded to pulmonologists between April 10, 2017, and May 17, 2017. Pulmonologists were included if they spent >20% of their time in direct patient care and had ≥5 patients with IPF receiving antifibrotics. Participants answered questions regarding timing and reasons for considering the initiation of antifibrotic therapy after an IPF diagnosis. A total of 169 pulmonologists participated. The majority (81.7%) considered initiating antifibrotic therapy immediately after IPF diagnosis all or most of the time (immediate group), while 18.3% considered it only some of the time or not at all (delayed group). Pulmonologists in the immediate group were more likely to work in private practice (26.1%), have a greater mean percentage of patients receiving antifibrotic therapy (60.8%), and decide to initiate treatment themselves (31.2%) versus those in the delayed group (16.1%, 30.5%, and 16.1%, respectively). Most pulmonologists consider initiating antifibrotic treatment immediately after establishing an IPF diagnosis all or most of the time versus using a "watch-and-wait" approach. Distinguishing characteristics between pulmonologists in the immediate group versus the delayed group included practice setting, percentage of patients receiving antifibrotic therapy, and the decision-making dynamics between the patient and the pulmonologist.
Keywords: Antifibrotic therapy; idiopathic pulmonary fibrosis.
Conflict of interest statement
Figures
References
-
- Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 431–440. - PubMed
-
- Genentech I. Esbriet (pirfenidone) capsules and film-coated tablets, for oral use [package insert]. South San Francisco, 2017.
-
- Boehringer I. Ofev (nintedanib) capsules, for oral use [package insert]. Ridgefield, 2018.
-
- Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192: e3–e19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

