DT-0111: a novel drug-candidate for the treatment of COPD and chronic cough
- PMID: 31558105
- PMCID: PMC6767719
- DOI: 10.1177/1753466619877960
DT-0111: a novel drug-candidate for the treatment of COPD and chronic cough
Abstract
Background: Extracellular adenosine 5'-triphosphate (ATP) plays important mechanistic roles in pulmonary disorders in general and chronic obstructive pulmonary disease (COPD) and cough in particular. The effects of ATP in the lungs are mediated to a large extent by P2X2/3 receptors (P2X2/3R) localized on vagal sensory nerve terminals (both C and Aδ fibers). The activation of these receptors by ATP triggers a pulmonary-pulmonary central reflex, which results in bronchoconstriction and cough, and is also proinflammatory due to the release of neuropeptides from these nerve terminals via the axon reflex. These actions of ATP in the lungs constitute a strong rationale for the development of a new class of drugs targeting P2X2/3R. DT-0111 is a novel, small, water-soluble molecule that acts as an antagonist at P2X2/3R sites.
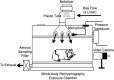
Methods: Experiments using receptor-binding functional assays, rat nodose ganglionic cells, perfused innervated guinea pig lung preparation ex vivo, and anesthetized and conscious guinea pigs in vivo were performed.
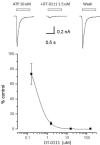
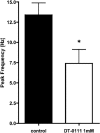
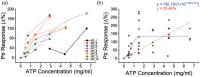
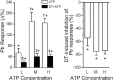
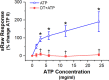
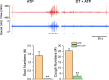
Results: DT-0111 acted as a selective and effective antagonist at P2X2/3R, that is, it did not activate or block P2YR; markedly inhibited the activation by ATP of nodose pulmonary vagal afferents in vitro; and, given as an aerosol, inhibited aerosolized ATP-induced bronchoconstriction and cough in vivo.
Conclusions: These results indicate that DT-0111 is an attractive drug-candidate for the treatment of COPD and chronic cough, both of which still constitute major unmet clinical needs. The reviews of this paper are available via the supplementary material section.
Keywords: ATP; COPD; asthma; bronchoconstriction; pulmonary inflammation; pulmonary-pulmonary reflex.
Conflict of interest statement
Figures

Similar articles
-
DT-0111: a novel P2X3 receptor antagonist.Purinergic Signal. 2023 Sep;19(3):467-479. doi: 10.1007/s11302-023-09930-5. Epub 2023 Mar 22. Purinergic Signal. 2023. PMID: 36944825 Free PMC article.
-
BLU-5937: A selective P2X3 antagonist with potent anti-tussive effect and no taste alteration.Pulm Pharmacol Ther. 2019 Jun;56:56-62. doi: 10.1016/j.pupt.2019.03.007. Epub 2019 Mar 20. Pulm Pharmacol Ther. 2019. PMID: 30902655
-
Update on novel purinergic P2X3 and P2X2/3 receptor antagonists and their potential therapeutic applications.Expert Opin Ther Pat. 2019 Dec;29(12):943-963. doi: 10.1080/13543776.2019.1693542. Epub 2019 Nov 20. Expert Opin Ther Pat. 2019. PMID: 31726893 Review.
-
P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex.J Appl Physiol (1985). 2010 Nov;109(5):1416-23. doi: 10.1152/japplphysiol.00774.2010. Epub 2010 Aug 26. J Appl Physiol (1985). 2010. PMID: 20798273 Free PMC article.
-
Extracellular Adenosine 5'-Triphosphate in Obstructive Airway Diseases.Chest. 2016 Oct;150(4):908-915. doi: 10.1016/j.chest.2016.06.045. Epub 2016 Aug 25. Chest. 2016. PMID: 27568579 Review.
Cited by
-
DT-0111: a novel P2X3 receptor antagonist.Purinergic Signal. 2023 Sep;19(3):467-479. doi: 10.1007/s11302-023-09930-5. Epub 2023 Mar 22. Purinergic Signal. 2023. PMID: 36944825 Free PMC article.
-
Testosterone Enhances KV Currents and Airway Smooth Muscle Relaxation Induced by ATP and UTP through P2Y4 Receptors and Adenylyl Cyclase Pathway.Int J Mol Sci. 2024 Apr 24;25(9):4652. doi: 10.3390/ijms25094652. Int J Mol Sci. 2024. PMID: 38731872 Free PMC article.
-
Degranulation of human mast cells: modulation by P2 receptors' agonists.Front Immunol. 2023 Oct 5;14:1216580. doi: 10.3389/fimmu.2023.1216580. eCollection 2023. Front Immunol. 2023. PMID: 37868982 Free PMC article. Review.
-
Oxidative Imbalance as a Crucial Factor in Inflammatory Lung Diseases: Could Antioxidant Treatment Constitute a New Therapeutic Strategy?Oxid Med Cell Longev. 2021 Feb 9;2021:6646923. doi: 10.1155/2021/6646923. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628371 Free PMC article. Review.
-
Intralaryngeal application of ATP evokes apneic response mainly via acting on P2X3 (P2X2/3) receptors of the superior laryngeal nerve in postnatal rats.J Appl Physiol (1985). 2021 Sep 1;131(3):986-996. doi: 10.1152/japplphysiol.00091.2021. Epub 2021 Jul 29. J Appl Physiol (1985). 2021. PMID: 34323594 Free PMC article.
References
-
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res 2001; 26: 959–969. - PubMed
-
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 2000; 362: 299–309. - PubMed
-
- Burnstock G, Brouns I, Adriaensen D, et al. Purinergic signaling in the airways. Pharmacol Rev 2012; 64: 834–868. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

