γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma
- PMID: 31558469
- PMCID: PMC6871311
- DOI: 10.1182/blood.2019000050
γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma
Abstract
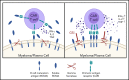
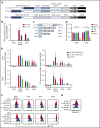
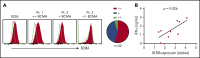
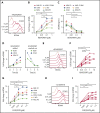
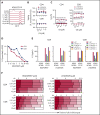
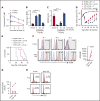
B-cell maturation antigen (BCMA) is a validated target for chimeric antigen receptor (CAR) T-cell therapy in multiple myeloma (MM). Despite promising objective response rates, most patients relapse, and low levels of BCMA on a subset of tumor cells has been suggested as a probable escape mechanism. BCMA is actively cleaved from the tumor cell surface by the ubiquitous multisubunit γ-secretase (GS) complex, which reduces ligand density on tumor cells for CAR T-cell recognition and releases a soluble BCMA (sBCMA) fragment capable of inhibiting CAR T-cell function. Sufficient sBCMA can accumulate in the bone marrow of MM patients to inhibit CAR T-cell recognition of tumor cells, and potentially limit efficacy of BCMA-directed adoptive T-cell therapy. We investigated whether blocking BCMA cleavage by small-molecule GS inhibitors (GSIs) could augment BCMA-targeted CAR T-cell therapy. We found that exposure of myeloma cell lines and patient tumor samples to GSIs markedly increased surface BCMA levels in a dose-dependent fashion, concurrently decreased sBCMA concentrations, and improved tumor recognition by CAR T cells in vitro. GSI treatment of MM tumor-bearing NOD/SCID/γc-/- mice increased BCMA expression on tumor cells, decreased sBCMA in peripheral blood, and improved antitumor efficacy of BCMA-targeted CAR T-cell therapy. Importantly, short-term GSI administration to MM patients markedly increases the percentage of BCMA+ tumor cells, and the levels of BCMA surface expression in vivo. Based on these data, a US Food and Drug Administration (FDA)-approved clinical trial has been initiated, combining GSI with concurrent BCMA CAR T-cell therapy. This trial was registered at www.clinicaltrials.gov as #NCT03502577.
© 2019 by The American Society of Hematology.
Figures


References
-
- Attal M, Harousseau JL, Stoppa AM, et al. . A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97. - PubMed
-
- Barlogie B, Kyle RA, Anderson KC, et al. . Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321 [published correction appears in J Clin Oncol. 2006;24(17):2687]. J Clin Oncol. 2006;24(6):929-936. - PubMed
-
- Bladé J, Rosiñol L, Cibeira MT, Rovira M, Carreras E. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115(18):3655-3663. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. ; Medical Research Council Adult Leukaemia Working Party . High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875-1883. - PubMed
-
- Harousseau JL, Attal M, Divine M, et al. . Autologous stem cell transplantation after first remission induction treatment in multiple myeloma. A report of the French Registry on Autologous Transplantation in Multiple Myeloma. Stem Cells. 1995;13(suppl 2):132-139. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

