Bevacizumab Reduces Permeability and Concurrent Temozolomide Delivery in a Subset of Patients with Recurrent Glioblastoma
- PMID: 31558474
- PMCID: PMC7139851
- DOI: 10.1158/1078-0432.CCR-19-1739
Bevacizumab Reduces Permeability and Concurrent Temozolomide Delivery in a Subset of Patients with Recurrent Glioblastoma
Abstract
Purpose: Targeting tumor blood vessels is an attractive therapy in glioblastoma (GBM), but the mechanism of action of these agents and how they modulate delivery of concomitant chemotherapy are not clear in humans. We sought to elucidate how bevacizumab modulates tumor vasculature and the impact those vascular changes have on drug delivery in patients with recurrent GBM.
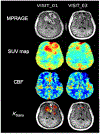
Experimental design: Temozolomide was labeled with [11C], and serial PET-MRI scans were performed in patients with recurrent GBM treated with bevacizumab and daily temozolomide. PET-MRI scans were performed prior to the first bevacizumab dose, 1 day after the first dose, and prior to the third dose of bevacizumab. We calculated tumor volume, vascular permeability (K trans), perfusion (cerebral blood flow), and the standardized uptake values (SUV) of [11C] temozolomide within the tumor.
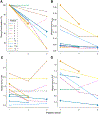
Results: Twelve patients were enrolled, resulting in 23 evaluable scans. Within the entire contrast-enhancing tumor volume, both temozolomide uptake and vascular permeability decreased after initiation of bevacizumab in most patients, whereas change in perfusion was more variable. In subregions of the tumor where permeability was low and the blood-brain barrier not compromised, increased perfusion correlated with increased temozolomide uptake.
Conclusions: Bevacizumab led to a decrease in permeability and concomitant delivery of temozolomide. However, in subregions of the tumor where permeability was low, increased perfusion improved delivery of temozolomide, suggesting that perfusion may modulate the delivery of chemotherapy in certain settings. These results support exploring whether lower doses of bevacizumab improve perfusion and concomitant drug delivery.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
K.E. Emblem reports intellectual property rights at NordicNeuroLab AS in Bergen, Norway. D.G. Duda is an employee/paid consultant for Bayer, Tilos, twoXAR, and BMS, and reports receiving commercial research grants from Bayer, Exelixis, BMS, Leap, and Merrimack. R.K. Jain is an employee/paid consultant for Chugai, holds ownership interest (including patents) at Enlight and SynDevRx, and is an advisory board member/unpaid consultant for Ophthotech, SPARC, SynDevRx, XTuit, Merck, the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. T. Batchelor is an employee/paid consultant for Genomicare, and reports receiving other commercial research support from UpToDate, Inc., Oakstone Publishing, Oncology Audio Digest, Champions Biotechnology, Amgen, NXDC, Upsher Smith, and Merck. All other authors declare no potential conflicts of interest by the other authors.
Figures


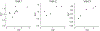
References
-
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002;20:4368–80. - PubMed
-
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–22. - PubMed
-
- Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:2048–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

