Clinical Assessment of 5-Fluorouracil/Leucovorin, Nab-Paclitaxel, and Irinotecan (FOLFIRABRAX) in Untreated Patients with Gastrointestinal Cancer Using UGT1A1 Genotype-Guided Dosing
- PMID: 31558477
- PMCID: PMC6942629
- DOI: 10.1158/1078-0432.CCR-19-1483
Clinical Assessment of 5-Fluorouracil/Leucovorin, Nab-Paclitaxel, and Irinotecan (FOLFIRABRAX) in Untreated Patients with Gastrointestinal Cancer Using UGT1A1 Genotype-Guided Dosing
Abstract
Purpose: 5-Fluorouracil (5-FU)/leucovorin, irinotecan, and nab-paclitaxel are all active agents in gastrointestinal cancers; the combination, FOLFIRABRAX, has not been previously evaluated. UDP Glucuronosyltransferase 1A1 (UGT1A1) clears SN-38, the active metabolite of irinotecan. UGT1A1*28 polymorphism reduces UGT1A1 enzymatic activity and predisposes to toxicity. We performed a trial to assess the safety and tolerability of FOLFIRABRAX with UGT1A1 genotype-guided dosing of irinotecan.
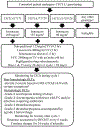
Patients and methods: Patients with previously untreated, advanced gastrointestinal cancers received FOLFIRABRAX with prophylactic pegfilgrastim every 14 days. UGT1A1 *1/*1, *1/*28, and *28/*28 patients received initial irinotecan doses of 180, 135, and 90 mg/m2, respectively. 5-FU 2,400 mg/m2 over 46 hours, leucovorin 400 mg/m2, and nab-paclitaxel 125 mg/m2 were administered. Doses were deemed tolerable if the dose-limiting toxicity (DLT) rate during cycle 1 was ≤35% in each genotype group. DLTs were monitored using a sequential procedure.
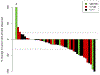
Results: Fifty patients enrolled, 30 pancreatic, 9 biliary tract, 6 gastroesophageal, and 5 others. DLTs occurred in 5 of 23 (22%) *1/*1 patients, 1 of 19 (5%) *1/*28 patients, and 0 of 7 *28/*28 patients. DLTs were all grade 3: diarrhea (3 patients), nausea (2 patients), and febrile neutropenia (1 patient). The overall response rate was 31%. Response rates in pancreatic, gastroesophageal, and biliary tract cancers were 34%, 50%, and 11%, respectively. Eighteen patients (36%) received therapy for at least 24 weeks.
Conclusions: FOLFIRABRAX with genotype-guided dosing of irinotecan is tolerable in patients with advanced gastrointestinal cancer and UGT1A1*1*1 or UGT1A1*1*28 genotypes. Too few *28/*28 patients were enrolled to provide conclusive results. Responses occurred across multiple tumor types.
©2019 American Association for Cancer Research.
Figures

Comment in
-
5-FU, Irinotecan, Nab-Paclitaxel in Gastrointestinal Cancers-Letter.Clin Cancer Res. 2020 Jul 15;26(14):3889. doi: 10.1158/1078-0432.CCR-20-0156. Clin Cancer Res. 2020. PMID: 32669271 No abstract available.
-
5-FU, Irinotecan, Nab-Paclitaxel in Gastrointestinal Cancers-Response.Clin Cancer Res. 2020 Jul 15;26(14):3890. doi: 10.1158/1078-0432.CCR-20-1219. Clin Cancer Res. 2020. PMID: 32669272 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

