A Multicenter Pilot Study on the Clinical Utility of Computational Modeling for Flow-Diverter Treatment Planning
- PMID: 31558504
- PMCID: PMC7028542
- DOI: 10.3174/ajnr.A6222
A Multicenter Pilot Study on the Clinical Utility of Computational Modeling for Flow-Diverter Treatment Planning
Abstract
Background and purpose: Selection of the correct flow-diverter size is critical for cerebral aneurysm treatment success, but it remains challenging due to the interplay of device size, anatomy, and deployment. Current convention does not address these challenges well. The goals of this pilot study were to determine whether computational modeling improves flow-diverter sizing over current convention and to validate simulated deployments.
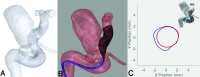
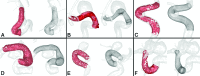
Materials and methods: Seven experienced neurosurgeons and interventional neuroradiologists used computational modeling to prospectively plan 19 clinical interventions. In each patient case, physicians simulated 2-4 flow-diverter sizes that were under consideration based on preprocedural imaging. In addition, physicians identified a preferred device size using the current convention. A questionnaire on the impact of computational modeling on the procedure was completed immediately after treatment. Rotational angiography image data were acquired after treatment and compared with flow-diverter simulations to validate the output of the software platform.
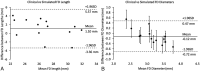
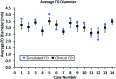
Results: According to questionnaire responses, physicians found the simulations useful for treatment planning, and they increased their confidence in device selection in 94.7% of cases. After viewing the simulations results, physicians selected a device size that was different from the original conventionally planned device size in 63.2% of cases. The average absolute difference between clinical and simulated flow-diverter lengths was 2.1 mm. In 57% of cases, average simulated flow-diverter diameters were within the measurement uncertainty of clinical flow-diverter diameters.
Conclusions: Physicians found computational modeling to be an impactful and useful tool for flow-diverter treatment planning. Validation results showed good agreement between simulated and clinical flow-diverter diameters and lengths.
© 2019 by American Journal of Neuroradiology.
Figures
Similar articles
-
Hemodynamics investigation for a giant aneurysm treated by a flow diverter implantation.Biomed Mater Eng. 2015;26 Suppl 1:S225-31. doi: 10.3233/BME-151309. Biomed Mater Eng. 2015. PMID: 26406006
-
Computational hemodynamics analysis of intracranial aneurysms treated with flow diverters: correlation with clinical outcomes.AJNR Am J Neuroradiol. 2014 Jan;35(1):136-42. doi: 10.3174/ajnr.A3790. Epub 2013 Nov 28. AJNR Am J Neuroradiol. 2014. PMID: 24287091 Free PMC article.
-
High-fidelity virtual stenting: modeling of flow diverter deployment for hemodynamic characterization of complex intracranial aneurysms.J Neurosurg. 2015 Oct;123(4):832-40. doi: 10.3171/2014.11.JNS14497. Epub 2015 Jun 19. J Neurosurg. 2015. PMID: 26090829 Free PMC article.
-
Flow diverter treatment of intracranial vertebral artery dissecting pseudoaneurysms.J Neurointerv Surg. 2017 Nov;9(11):1064-1068. doi: 10.1136/neurintsurg-2017-013020. Epub 2017 Apr 24. J Neurointerv Surg. 2017. PMID: 28438894 Review.
-
Virtual simulation for flow-diverter selection and sizing in the endovascular treatment of intracranial aneurysms: A systematic review and meta-analysis.Interv Neuroradiol. 2025 Mar 2:15910199251323006. doi: 10.1177/15910199251323006. Online ahead of print. Interv Neuroradiol. 2025. PMID: 40025753 Free PMC article. Review.
Cited by
-
Cerebral Aneurysm Occlusion at 12-Month Follow-Up After Flow-Diverter Treatment: Statistical Modeling for V&V With Real-World Data.Front Med Technol. 2021 Sep 17;3:705003. doi: 10.3389/fmedt.2021.705003. eCollection 2021. Front Med Technol. 2021. PMID: 35047944 Free PMC article.
-
Virtual and augmented reality for biomedical applications.Cell Rep Med. 2021 Jul 21;2(7):100348. doi: 10.1016/j.xcrm.2021.100348. eCollection 2021 Jul 20. Cell Rep Med. 2021. PMID: 34337564 Free PMC article. Review.
-
Association Between Aneurysmal Haemodynamics and Device Microstructural Characteristics After Flow-Diversion Treatments With Dual Stents of Different Sizes: A Numerical Study.Front Physiol. 2021 May 25;12:663668. doi: 10.3389/fphys.2021.663668. eCollection 2021. Front Physiol. 2021. PMID: 34113263 Free PMC article.
-
Semi-automated cerebral aneurysm segmentation and geometric analysis for WEB sizing utilizing a cloud-based computational platform.Interv Neuroradiol. 2021 Dec;27(6):828-836. doi: 10.1177/15910199211009111. Epub 2021 Apr 7. Interv Neuroradiol. 2021. PMID: 33823619 Free PMC article.
References
-
- U.S. Food and Drug Administration. P100018/S015, PipelineTM, Flex Embolization Device approval letter. https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100018S015A.pdf. Accessed May 22, 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
