N-Glycosylation is required for secretion of the precursor to brain-derived neurotrophic factor (proBDNF) carrying sulfated LacdiNAc structures
- PMID: 31558607
- PMCID: PMC6851326
- DOI: 10.1074/jbc.RA119.009989
N-Glycosylation is required for secretion of the precursor to brain-derived neurotrophic factor (proBDNF) carrying sulfated LacdiNAc structures
Abstract
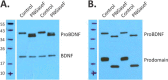
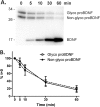
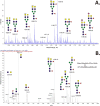
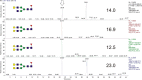
Brain-derived neurotrophic factor (BDNF) is generated by proteolytic cleavage of a prodomain from the proBDNF precursor either intracellularly by furin-like proteases or extracellularly by plasmin or matrix metalloproteinases. ProBDNF carries a single N-glycosylation sequon (Asn-127) that remains virtually unstudied despite being located in a highly conserved region proximal to the proteolytic site. To study the proBDNF structure and function, here we expressed the protein and its nonglycosylated N127Q mutant in HEK293F cells. We found that mutation of the Asn-127 prevents intracellular maturation and secretion, an effect reproduced in WT proBDNF by tunicamycin-induced inhibition of N-glycosylation. Absence of the N-glycan did not affect the kinetics of proBDNF cleavage by furin in vitro, indicating that effects other than a direct furin-proBDNF interaction may regulate proBDNF maturation. Using an optimized LC-MS/MS workflow, we demonstrate that secreted proBDNF is fully glycosylated and carries rare N-glycans terminated by GalNAcβ1-4GlcNAcβ1-R (LacdiNAc) extensively modified by terminal sulfation. We and others noted that this type of glycosylation is protein-specific, extends to proBDNF expressed in PC12 cells, and implies the presence of interacting partners that recognize this glycan epitope. The findings of our study reveal that proBDNF carries an unusual type of N-glycans important for its processing and secretion. Our results open new opportunities for functional studies of these protein glycoforms in different cells and tissues.
Keywords: LacdiNAc; N121Q; brain-derived neurotrophic factor (BDNF); glycoprotein; glycosylation; mass spectrometry (MS); maturation; neurotrophin; sulfation.
© 2019 Benicky et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

