Heat resilience in embryonic zebrafish revealed using an in vivo stress granule reporter
- PMID: 31558681
- PMCID: PMC6826007
- DOI: 10.1242/jcs.234807
Heat resilience in embryonic zebrafish revealed using an in vivo stress granule reporter
Abstract
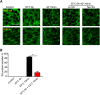
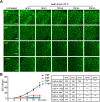
Although the regulation of stress granules has become an intensely studied topic, current investigations of stress granule assembly, disassembly and dynamics are mainly performed in cultured cells. Here, we report the establishment of a stress granule reporter to facilitate the real-time study of stress granules in vivo Using CRISPR/Cas9, we fused a green fluorescence protein (GFP) to endogenous G3BP1 in zebrafish. The GFP-G3BP1 reporter faithfully and robustly responded to heat stress in zebrafish embryos and larvae. The induction of stress granules varied by brain regions under the same stress condition, with the midbrain cells showing the highest efficiency and dynamics. Furthermore, pre-conditioning using lower heat stress significantly limited stress granule formation during subsequent higher heat stress. More interestingly, stress granule formation was much more robust in zebrafish embryos than in larvae and coincided with significantly elevated levels of phosphorylated eIF2α and enhanced heat resilience. Therefore, these findings have generated new insights into stress response in zebrafish during early development and demonstrated that the GFP-G3BP1 knock-in zebrafish could be a valuable tool for the investigation of stress granule biology.This article has an associated First Person interview with the first author of the paper.
Keywords: Early development; G3BP1; Heat shock; In vivo reporter; Stress granule; Stress resilience; Zebrafish.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsJ.X., R.W., H.Z., and J.D. are co-inventors on the patent application ‘The method for the establishment of a zebrafish model for stress granule research and its use’, application number CN2019102240623 filed with the China National Intellectual Property Administration.
Figures




References
-
- Apicco D. J., Ash P. E. A., Maziuk B., LeBlang C., Medalla M., Al Abdullatif A., Ferragud A., Botelho E., Ballance H. I., Dhawan U. et al. (2018). Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat. Neurosci. 21, 72-80. 10.1038/s41593-017-0022-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

