The hidden structure of human enamel
- PMID: 31558712
- PMCID: PMC6763454
- DOI: 10.1038/s41467-019-12185-7
The hidden structure of human enamel
Abstract
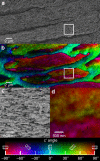
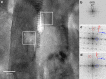
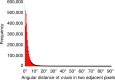
Enamel is the hardest and most resilient tissue in the human body. Enamel includes morphologically aligned, parallel, ∼50 nm wide, microns-long nanocrystals, bundled either into 5-μm-wide rods or their space-filling interrod. The orientation of enamel crystals, however, is poorly understood. Here we show that the crystalline c-axes are homogenously oriented in interrod crystals across most of the enamel layer thickness. Within each rod crystals are not co-oriented with one another or with the long axis of the rod, as previously assumed: the c-axes of adjacent nanocrystals are most frequently mis-oriented by 1°-30°, and this orientation within each rod gradually changes, with an overall angle spread that is never zero, but varies between 30°-90° within one rod. Molecular dynamics simulations demonstrate that the observed mis-orientations of adjacent crystals induce crack deflection. This toughening mechanism contributes to the unique resilience of enamel, which lasts a lifetime under extreme physical and chemical challenges.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Lawn BR, Lee JJ-W, Chai H. Teeth: among nature’s most durable biocomposites. Ann. Rev. Mater. Res. 2010;40:55–75. doi: 10.1146/annurev-matsci-070909-104537. - DOI

