Electrochemical Redox Refrigeration
- PMID: 31558735
- PMCID: PMC6763465
- DOI: 10.1038/s41598-019-50118-y
Electrochemical Redox Refrigeration
Abstract
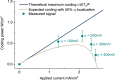
The high conformational entropy change of the Fe(CN)63-/4- redox reaction can be used as the basis for a compact electrochemical refrigerator. This device is comparable to a liquid version of a Peltier cooler, with two distinct advantages: (1) the entropy change per carrier (1.5 mV/K) of the electrochemical refrigerant is more than 5 times larger than that of state-of-the-art solid thermoelectric materials; and (2) the liquid electrolyte can be advected continuously away from the cooling junction, so that Joule heating in the bulk element does not diminish the delivered cooling effect. In this work, we use infrared microscopy to visualize the thermal aspects of Fe(CN)63-/4- redox, and compare the estimated cooling to calculated values with and without electrolyte flow. While the temperature differences achieved in a single cell are small (~50 mK) and not enhanced by electrolyte flow, the cooling power density (~0.5 W/cm3) is high when normalized to the small electrode volume. Non-dimensional figures of merit are proposed to identify electrochemical redox species for maximizing the cooling effect.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- U.S. Energy Information Administration. Annual Energy Outlook, https://www.eia.gov/outlooks/aeo/ (2019).
-
- United Nations Population Division. World Population Prospects, 2017 Revision: Key Findings and Advance Tables, https://population.un.org/wpp/Publications/ (2019).
-
- California Air Resources Board. High-GWP Refrigerants, https://ww2.arb.ca.gov/resources/documents/high-gwp-refrigerants (2019).
-
- United Nations Treaty Collection. Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer, https://treaties.un.org/Pages/Home.aspx?clang=_en (2016). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

