Thermally Stable Donor-Acceptor Type (Alkynyl)Gold(III) TADF Emitters Achieved EQEs and Luminance of up to 23.4% and 70 300 cd m-2 in Vacuum-Deposited OLEDs
- PMID: 31559124
- PMCID: PMC6755518
- DOI: 10.1002/advs.201802297
Thermally Stable Donor-Acceptor Type (Alkynyl)Gold(III) TADF Emitters Achieved EQEs and Luminance of up to 23.4% and 70 300 cd m-2 in Vacuum-Deposited OLEDs
Abstract
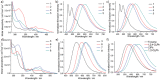
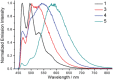
Thermally stable, strongly luminescent gold-TADF emitters are the clue to realize practical applications of gold metal in next generation display and lighting technology, a scarce example of which is herein described. A series of donor-acceptor type cyclometalated gold(III) alkynyl complexes with some of them displaying highly efficient thermally activated delayed fluorescence (TADF) with Φ up to 88% in thin films and emission lifetimes of ≈1-2 µs at room temperature are developed. The emission color of these complexes is readily tunable from green to red by varying the donor unit and cyclometalating ligand. Vacuum-deposited organic light-emitting diodes (OLEDs) with these complexes as emissive dopants achieve external quantum efficiencies (EQEs) and luminance of up to 23.4% and 70 300 cd m-2, respectively.
Keywords: gold; ligand‐to‐ligand charge transfer; organic light‐emitting devices; thermally activated delayed fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ma Y., Zhou X., Shen J., Chao H.‐Y., Che C.‐M., Appl. Phys. Lett. 1999, 74, 1361.
-
- Ma Y., Che C.‐M., Chao H.‐Y., Zhou X., Chan W.‐H., Shen J., Adv. Mater. 1999, 11, 852.
-
- Au V. K.‐M., Wong K. M.‐C., Tsang D. P.‐K., Chan M.‐Y., Zhu N., Yam V. W.‐W., J. Am. Chem. Soc. 2010, 132, 14273. - PubMed
-
- Cheng G., Chan K. T., To W.‐P., Che C.‐M., Adv. Mater. 2014, 26, 2540. - PubMed
-
- Chan K. T., Tong G. S. M., Wan Q., Cheng G., Yang C., Che C.‐M., Chem. ‐ Asian J. 2017, 12, 2104. - PubMed
LinkOut - more resources
Full Text Sources
