Perturbed Microbial Ecology in Myasthenia Gravis: Evidence from the Gut Microbiome and Fecal Metabolome
- PMID: 31559142
- PMCID: PMC6755540
- DOI: 10.1002/advs.201901441
Perturbed Microbial Ecology in Myasthenia Gravis: Evidence from the Gut Microbiome and Fecal Metabolome
Erratum in
-
Erratum: Perturbed Microbial Ecology in Myasthenia Gravis: Evidence from the Gut Microbiome and Fecal Metabolome.Adv Sci (Weinh). 2020 May 20;7(10):2001296. doi: 10.1002/advs.202001296. eCollection 2020 May. Adv Sci (Weinh). 2020. PMID: 32440491 Free PMC article.
Abstract
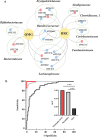
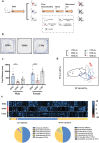
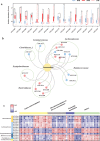
Myasthenia gravis (MG) is a devastating acquired autoimmune disease. Emerging evidence indicates that the gut microbiome plays a key role in maintaining immune system homeostasis. This work reports that MG is characterized by decreased α-phylogenetic diversity, and significantly disturbed gut microbiome and fecal metabolome. The altered gut microbial composition is associated with fecal metabolome changes, with 38.75% of altered bacterial operational taxonomic units showing significant correlations with a range of metabolite biomarkers. Some microbes are particularly linked with MG severity. Moreover, a combination of microbial makers and their correlated metabolites enable discriminating MG from healthy controls (HCs) with 100% accuracy. To investigate whether disturbed gut mcirobiome might contribute to the onset of MG, germ-free (GF) mice are initially colonized with MG microbiota (MMb) or healthy microbiota (HMb), and then immunized in a classic mouse model of MG. The MMb mice demonstrate substantially impaired locomotion ability compared with the HMb mice. This effect could be reversed by cocolonizing GF mice with both MMb and HMb. The MMb mice also exhibit similar disturbances of fecal metabolic pathways as found in MG. Together these data demonstrate disturbances in microbiome composition and activity that are likely to be relevant to the pathogenesis of MG.
Keywords: fecal microbiota transplantation; germ‐free mice; gut microbiome; metabolome; myasthenia gravis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gilhus N. E., N. Engl. J. Med. 2016, 375, 2570. - PubMed
-
- Gilhus N. E., Skeie G. O., Romi F., Lazaridis K., Zisimopoulou P., Tzartos S. Nat. Rev. Neurol. 2016, 12, 259. - PubMed
-
- Evoli A., Curr. Opin. Neurol. 2017, 30, 464. - PubMed
-
- Crisp S. J., Kullmann D. M., Vincent A., Nat. Rev. Neurosci. 2016, 17, 103. - PubMed
LinkOut - more resources
Full Text Sources
