Magnetic resonance imaging of adrenal gland: state of the art
- PMID: 31559189
- PMCID: PMC6755942
- DOI: 10.21037/gs.2019.06.02
Magnetic resonance imaging of adrenal gland: state of the art
Abstract
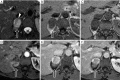
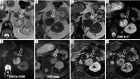
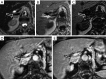
Detection of adrenal lesions, because of the widespread use of imaging and especially high-resolution imaging procedures, is increased. Because of the importance to characterize those findings, magnetic resonance imaging (MRI), in particular chemical shift imaging (CSI), is useful to distinguish whether a lesion is benignant or malignant and to avoid further diagnostic or surgical procedures. It represents the first choice of imaging in patient like children or pregnant women, and a valid complement to other imaging techniques like CT or PET/CT. In this review we analyze the role and characteristic of MRI and the imaging features of most common benignant (adenoma, hyperplasia, pheochromocytoma, hemorrhage, cyst, myelolipoma, teratoma, ganglioneuroma, cystic lymphangioma, hemangioma) and malignant [neuroblastoma, adrenocortical carcinoma (ACC), metastases, lymphoma] adrenal lesions.
Keywords: Imaging; adrenal glands; magnetic resonance imaging (MRI).
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
