Long-term outcomes of neoadjuvant-synchronous S-1 plus radiotherapy for locally advanced rectal cancer: a multi-institutional prospective phase II study
- PMID: 31559360
- PMCID: PMC6752138
- DOI: 10.23922/jarc.2018-011
Long-term outcomes of neoadjuvant-synchronous S-1 plus radiotherapy for locally advanced rectal cancer: a multi-institutional prospective phase II study
Abstract
Objectives: This study aimed to evaluate the long-term outcomes of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced rectal cancer.
Methods: A multi-institutional, prospective, phase II trial was conducted between April 2009 and August 2011. The study enrolled 37 patients with histologically proven rectal carcinoma (T3-4 N0-3 M0) who underwent neoadjuvant chemoradiotherapy with S-1. Total mesorectal excision with D3 lymphadenectomy was performed 4-8 weeks after completion of neoadjuvant chemoradiotherapy with S-1 in 36 patients. We then analyzed late adverse events, overall survival, and disease-free survival.
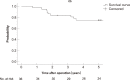
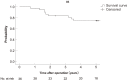
Results: The median patient age was 59 years (range: 32-79 years); there were 24 men and 13 women. Ten patients had Stage II disease, and 27 had Stage III disease. Severe late adverse events occurred in 7 patients (18.9%). The 5-year disease-free survival was 66.7%, and the 5-year overall survival was 74.7%. The median follow-up period was 57 months. Local recurrences developed in 5 patients (13.5%), and distant metastases developed in 8 (21.6%).
Conclusion: Neoadjuvant-synchronous chemoradiotherapy with S-1 for locally advanced rectal cancer is feasible in terms of adverse events and long-term outcomes. (UMIN Clinical Trial Registry: UMIN000003396).
Keywords: S-1; neoadjuvant chemoradiotherapy; rectal cancer.
Conflict of interest statement
Conflicts of Interest There are no conflicts of interest.
Figures
Similar articles
-
A prospective feasibility study to evaluate neoadjuvant-synchronous S-1 with radiotherapy for locally advanced rectal cancer: A multicentre phase II trial.Mol Clin Oncol. 2016 Apr;4(4):510-514. doi: 10.3892/mco.2016.767. Epub 2016 Feb 4. Mol Clin Oncol. 2016. PMID: 27073652 Free PMC article.
-
[Analysis on efficacy and safety of total neoadjuvant therapy in patients with locally advanced rectal cancer with high risk factors].Zhonghua Wei Chang Wai Ke Za Zhi. 2019 Apr 25;22(4):349-356. doi: 10.3760/cma.j.issn.1671-0274.2019.04.007. Zhonghua Wei Chang Wai Ke Za Zhi. 2019. PMID: 31054549 Chinese.
-
Prospective feasibility study to evaluate neoadjuvant-synchronous S-1 + RT for locally advanced rectal cancer: a multicenter phase II trial (UMIN ID: 03396).Jpn J Clin Oncol. 2013 Mar;43(3):321-3. doi: 10.1093/jjco/hys219. Epub 2012 Dec 28. Jpn J Clin Oncol. 2013. PMID: 23275647 Clinical Trial.
-
Neoadjuvant oxaliplatin and capecitabine combined with bevacizumab plus radiotherapy for locally advanced rectal cancer: results of a single-institute phase II study.Cancer Commun (Lond). 2018 May 21;38(1):24. doi: 10.1186/s40880-018-0294-z. Cancer Commun (Lond). 2018. PMID: 29784042 Free PMC article. Clinical Trial.
-
Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer.Br J Surg. 2019 Jul;106(8):979-987. doi: 10.1002/bjs.11171. Epub 2019 May 10. Br J Surg. 2019. PMID: 31074508
Cited by
-
Short-term outcomes of preoperative chemoradiotherapy with S-1 for locally advanced rectal cancer.Mol Clin Oncol. 2021 Jan;14(1):4. doi: 10.3892/mco.2020.2166. Epub 2020 Nov 3. Mol Clin Oncol. 2021. PMID: 33235732 Free PMC article.
-
Efficacy and safety of preoperative chemoradiotherapy with S-1 for advanced rectal cancer: a phase II study.Rep Pract Oncol Radiother. 2023 Apr 6;28(1):36-46. doi: 10.5603/RPOR.a2023.0005. eCollection 2023. Rep Pract Oncol Radiother. 2023. PMID: 37122915 Free PMC article.
-
Efficacy and tolerability of preoperative chemoradiotherapy with S-1 alone for locally advanced rectal cancer.J Radiat Res. 2021 Mar 10;62(2):300-308. doi: 10.1093/jrr/rraa117. J Radiat Res. 2021. PMID: 33341902 Free PMC article.
-
Efficacy of concurrent chemoradiotherapy with thalidomide and S-1 for esophageal carcinoma and its influence on serum tumor markers.World J Gastrointest Oncol. 2023 Jul 15;15(7):1262-1270. doi: 10.4251/wjgo.v15.i7.1262. World J Gastrointest Oncol. 2023. PMID: 37546558 Free PMC article.
References
-
- Cancer Registry and Statistics. Japan ed. Cancer Information Service NCC; 2013.
-
- Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006 Nov; 49: 1663-72. - PubMed
-
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006 Sep; 355: 1114-23. - PubMed
-
- Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006 Oct; 24: 4620-5. - PubMed
-
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011 Jul; 29: 2773-80. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous