Gut microbiota regulates cardiac ischemic tolerance and aortic stiffness in obesity
- PMID: 31559829
- PMCID: PMC6960779
- DOI: 10.1152/ajpheart.00346.2019
Gut microbiota regulates cardiac ischemic tolerance and aortic stiffness in obesity
Abstract
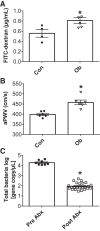
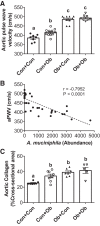
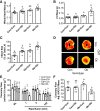
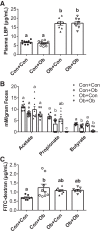
The gut microbiota has emerged as an important regulator of host physiology, with recent data suggesting a role in modulating cardiovascular health. The present study determined if gut microbial signatures could transfer cardiovascular risk phenotypes between lean and obese mice using cecal microbiota transplantation (CMT). Pooled cecal contents collected from obese leptin-deficient (Ob) mice or C57Bl/6j control (Con) mice were transplanted by oral gavage into cohorts of recipient Ob and Con mice maintained on identical low-fat diets for 8 wk (n = 9-11/group). Cardiovascular pathology was assessed as the degree of arterial stiffness (aortic pulse wave velocity) and myocardial infarct size following a 45/120 min ex vivo global cardiac ischemia-reperfusion protocol. Gut microbiota was characterized by 16S rDNA sequencing, along with measures of intestinal barrier function and cecal short-chain fatty acid (SCFA) composition. Following CMT, the gut microbiota of recipient mice was altered to resemble that of the donors. Ob CMT to Con mice increased arterial stiffness, left ventricular (LV) mass, and myocardial infarct size, which were associated with greater gut permeability and reduced cecal SCFA concentrations. Conversely, Con CMT to Ob mice increased cecal SCFA, reduced LV mass, and attenuated myocardial infarct size, with no effects on gut permeability or arterial stiffness. Collectively, these data demonstrate that obesity-related changes in the gut microbiota, independent of dietary manipulation, regulate hallmark measures of cardiovascular pathology in mice and highlight the potential of microbiota-targeted therapeutics for reducing cardiovascular pathology and risk in obesity.NEW & NOTEWORTHY These data are the first to demonstrate that cecal microbiota transplantation (CMT) can alter cardiovascular pathology in lean and obese mice independent from alterations in dietary intake. Myocardial infarct size was reduced in obese mice receiving lean CMT and worsened in lean mice receiving obese CMT. Lean mice receiving obese CMT also displayed increased aortic stiffness. These changes were accompanied by alterations in short-chain fatty acids and gut permeability.
Keywords: arterial stiffness; cecal transplant; ischemia-reperfusion; microbiota; obesity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

