Viral Kinetics and Resistance Development in Children Treated with Neuraminidase Inhibitors: The Influenza Resistance Information Study (IRIS)
- PMID: 31560055
- PMCID: PMC7442852
- DOI: 10.1093/cid/ciz939
Viral Kinetics and Resistance Development in Children Treated with Neuraminidase Inhibitors: The Influenza Resistance Information Study (IRIS)
Abstract
Background: We studied the effect of age, baseline viral load, vaccination status, antiviral therapy, and emergence of drug resistance on viral shedding in children infected with influenza A or B virus.
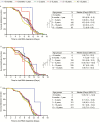
Methods: Samples from children (aged ≤13 years) enrolled during the 7 years of the prospective Influenza Resistance Information Study were analyzed using polymerase chain reaction to determine the influenza virus (sub-)type, viral load, and resistance mutations. Disease severity was assessed; clinical symptoms were recorded. The association of age with viral load and viral clearance was examined by determining the area under the curve for viral RNA shedding using logistic regression and Kaplan-Meier analyses.
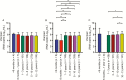
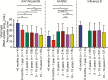
Results: A total of 2131 children infected with influenza (683, A/H1N1pdm09; 825, A/H3N2; 623, influenza B) were investigated. Age did not affect the mean baseline viral load. Children aged 1-5 years had prolonged viral RNA shedding (±1-2 days) compared with older children and up to 1.2-fold higher total viral burden. Besides, in older age (odds ratio [OR], 1.08; confidence interval [CI], 1.05-1.12), prior vaccination status (OR, 1.72; CI, 1.22-2.43) and antiviral treatment (OR, 1.74; CI, 1.43-2.12) increased the rate of viral clearance. Resistance mutations were detected in 49 children infected with influenza A virus (34, A/H1N1pdm09; 15, A/H3N2) treated with oseltamivir, most of whom were aged <5 years (n = 39).
Conclusions: Children aged 1-5 years had a higher total viral burden with prolonged virus shedding and had an increased risk of acquiring resistance mutations following antiviral treatment.
Clinical trials registration: NCT00884117.
Keywords: Influenza Resistance Information Study; influenza; pediatrics; resistance mutations; viral load.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Different drug-resistant influenza A(H3N2) variants in two immunocompromised patients treated with oseltamivir during the 2011-2012 influenza season in Italy.J Clin Virol. 2013 Sep;58(1):132-7. doi: 10.1016/j.jcv.2013.06.003. Epub 2013 Jun 27. J Clin Virol. 2013. PMID: 23810646
-
Viral shedding and susceptibility to oseltamivir in hospitalized immunocompromised patients with influenza in the Influenza Resistance Information Study (IRIS).Antivir Ther. 2015;20(6):633-42. doi: 10.3851/IMP2957. Epub 2015 Apr 7. Antivir Ther. 2015. PMID: 25849228 Clinical Trial.
-
Global assessment of resistance to neuraminidase inhibitors, 2008-2011: the Influenza Resistance Information Study (IRIS).Clin Infect Dis. 2013 May;56(9):1197-205. doi: 10.1093/cid/cis1220. Epub 2013 Jan 10. Clin Infect Dis. 2013. PMID: 23307766
-
Influenza virus susceptibility and resistance to oseltamivir.Antivir Ther. 2007;12(4 Pt B):603-16. Antivir Ther. 2007. PMID: 17944268 Review.
-
Influenza virus resistance to antiviral therapy.Adv Pharmacol. 2013;67:217-46. doi: 10.1016/B978-0-12-405880-4.00006-8. Adv Pharmacol. 2013. PMID: 23886002 Review.
Cited by
-
Guidance on the use of antiviral agents for the 2019-2020 influenza season.J Assoc Med Microbiol Infect Dis Can. 2020 Jun 23;5(2):57-60. doi: 10.3138/jammi.2020-01-13. eCollection 2020 Jun. J Assoc Med Microbiol Infect Dis Can. 2020. PMID: 36338179 Free PMC article. No abstract available.
-
Early Fever Resolution in Early Childhood Influenza Treated with Baloxavir Marboxil: A Retrospective Study Compared to Those with Oseltamivir.Medicina (Kaunas). 2023 Aug 25;59(9):1543. doi: 10.3390/medicina59091543. Medicina (Kaunas). 2023. PMID: 37763660 Free PMC article.
-
Antiviral Peptides as Anti-Influenza Agents.Int J Mol Sci. 2022 Sep 28;23(19):11433. doi: 10.3390/ijms231911433. Int J Mol Sci. 2022. PMID: 36232735 Free PMC article. Review.
-
Clinical and virological outcomes with baloxavir compared with oseltamivir in pediatric patients aged 6 to < 12 years with influenza: an open-label, randomized, active-controlled trial protocol.BMC Infect Dis. 2021 Aug 9;21(1):777. doi: 10.1186/s12879-021-06494-w. BMC Infect Dis. 2021. PMID: 34372769 Free PMC article.
-
Strategies and efforts in circumventing the emergence of antiviral resistance against conventional antivirals.NPJ Antimicrob Resist. 2025 Jun 9;3(1):54. doi: 10.1038/s44259-025-00125-z. NPJ Antimicrob Resist. 2025. PMID: 40490516 Free PMC article. Review.
References
-
- Fraaij PL, Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine 2011; 29:7524–8. - PubMed

