Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer
- PMID: 31560066
- PMCID: PMC6927320
- DOI: 10.1093/annonc/mdz397
Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer
Abstract
Background: In the SPARTAN study, compared with placebo, apalutamide added to ongoing androgen deprivation therapy significantly prolonged metastasis-free survival (MFS) and time to symptomatic progression in patients with high-risk non-metastatic castration-resistant prostate cancer (nmCRPC). Overall survival (OS) results at the first interim analysis (IA1) were immature, with 104 of 427 (24%) events required for planned final OS analysis. Here, we report the results of a second pre-specified interim analysis (IA2).
Methods: One thousand two hundred and seven patients with nmCRPC were randomized 2 : 1 to apalutamide (240 mg daily) or placebo. The primary end point of the study was MFS. Subsequent therapy for metastatic CRPC was permitted. When the primary end point was met, the study was unblinded. Patients receiving placebo who had not yet developed metastases were offered open-label apalutamide. At IA2, pre-specified analysis of OS was undertaken, using a group-sequential testing procedure with O'Brien-Fleming-type alpha spending function. Safety and second progression-free survival (PFS2) were assessed.
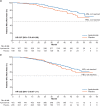
Results: Median follow-up was 41 months. With 285 (67% of required) OS events, apalutamide was associated with an improved OS compared with placebo (HR 0.75; 95% CI 0.59-0.96; P = 0.0197), although the P-value did not cross the pre-specified O'Brien-Fleming boundary of 0.0121. Apalutamide improved PFS2 (HR 0.55; 95% CI 0.45-0.68). At IA2, 69% of placebo-treated and 40% of apalutamide-treated patients had received subsequent life-prolonging therapy for metastatic CRPC. No new safety signals were observed.
Conclusion: In patients with nmCRPC, apalutamide was associated with a 25% reduction in risk of death compared with placebo. This OS benefit was observed despite crossover of placebo-treated patients and higher rates of subsequent life-prolonging therapy for the placebo group.
Keywords: apalutamide; non-metastatic castration-resistant prostate cancer; overall survival; subsequent therapy.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures


Comment in
-
Re: Apalutamide and Overall Survival in Non-metastatic Castration-resistant Prostate Cancer.Eur Urol. 2020 Jul;78(1):116-117. doi: 10.1016/j.eururo.2020.02.015. Epub 2020 Mar 12. Eur Urol. 2020. PMID: 32173126 No abstract available.
References
-
- Mateo J, Fizazi K, Gillessen S. et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol 2019; 75(2): 285–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

