Validation of a self-completed Dystonia Non-Motor Symptoms Questionnaire
- PMID: 31560179
- PMCID: PMC6801169
- DOI: 10.1002/acn3.50900
Validation of a self-completed Dystonia Non-Motor Symptoms Questionnaire
Abstract
Objective: To develop and validate a novel 14-item self-completed questionnaire (in English and German) enquiring about the presence of non-motor symptoms (NMS) during the past month in patients with craniocervical dystonia in an international multicenter study.
Methods: The Dystonia Non-Motor Symptoms Questionnaire (DNMSQuest) covers seven domains including sleep, autonomic symptoms, fatigue, emotional well-being, stigma, activities of daily living, sensory symptoms. The feasibility and clinimetric attributes were analyzed.
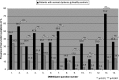
Results: Data from 194 patients with CD (65.6% female, mean age 58.96 ± 12.17 years, duration of disease 11.95 ± 9.40 years) and 102 age- and sex-matched healthy controls (66.7% female, mean age 55.67 ± 17.62 years) were collected from centres in Germany and the UK. The median total NMS score in CD patients was 5 (interquartile range 3-7), significantly higher than in healthy controls with 1 (interquartile range 0.75-2.25) (P < 0.001, Mann-Whitney U-test). Evidence for intercorrelation and convergent validity is shown by moderate to high correlations of total DNMSQuest score with motor symptom severity (TWSTRS: rs = 0.61), clinical global impression (rs = 0.40), and health-related quality of life measures: CDQ-24 (rs = 0.74), EQ-5D index (rs = -0.59), and scale (rs = -0.49) (all P < 0.001). Data quality and acceptability was very satisfactory.
Interpretation: The DNMSQuest, a patient self-completed questionnaire for NMS assessment in CD patients, appears robust, reproducible, and valid in clinical practice showing a tangible impact of NMS on quality of life in CD. As there is no specific, comprehensive, validated tool to assess the burden of NMS in dystonia, the DNMSQuest can bridge this gap and could easily be integrated into clinical practice.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
Funding sources for this study: None.
Lisa Klingelhoefer reports academic grants from EU Horizon 2020, honoraria for lectures from Berlin Partner für Wirtschaft und Technologie GmbH and Desitin.
K Ray Chaudhuri reports: Advisory board: AbbVie, UCB, Sunovion, Pfizer, Jazz Pharma, GKC, Bial, Cynapsus, Novartis, Lobsor, Stada, Medtronic, Zambon, Profile, Sunovion. Honoraria for lectures: AbbVie, Britannia, UCB, Mundipharma, Zambon, Novartis, Boeringer Ingelheim Neuroderm, Sunovion. Investigator initiated grants: Britania Pharmaceuticals, AbbVie, UCB, GKC, Bial. Aacdemic grants: EU, IMI EU, Parkinson's UK, NIHR, PDNMG, EU (Horizon 2020), Kirby Laing Foundation, NPF, MRC.
K Ray Chaudhuri and Anna Sauerbier acknowledge independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Christoph Kamm has received honoraria for presentations/lectures and travel grants from Merz Pharmaceuticals.
Pablo Martinez‐Martin: Honoraria: from National School of Public Health (ISCIII) and Editorial Viguera for lecturing in courses; International Parkinson and Movement Disorder Society for management of the Program on Rating Scales; Air Liquide, Abbvie, Zambon, and HM Hospitales de Madrid for advice in clinical‐epidemiological studies. License fee payments for the King’s Parkinson’s Disease Pain scale.
Kailash Bhatia: to be added.
Anna Sauerbier reports grants from Parkinson’s UK and Kirby Laing, consultancy and speaker related fees from Britannia, UCB and Bial.
Carmen Rodriguez‐Blazquez: financial support from Abbvie for attending national and international meetings.
Bettina Balint is supported by the Robert Bosch Foundation.
Robert Untucht received a grant from the Stiftung Hochschulmedizin (medical university foundation) in Dresden and discloses support by Merz Pharma and Ipsen for travel costs to conferences.
Davide Martino: Honoraria for consultancies from Allergan. Grant research support from Parkinson Association of Alberta.
Alexander Storch has received funding from the Deutsche Forschungsgemeinschaft (DFG) and the Helmholtz‐Association; received honoraria for presentations/lectures/consultancies or advisory boards from Desitin, Grünenthal, UCB, Messe Bremen, and AbbVie. He has served on the editorial boards of Stem Cells and Stem Cells International.Heinz Reichmann was acting on Advisory Boards and gave lectures and received research grants from Abbott, Abbvie, Bayer Health Care, Bial, Boehringer/Ingelheim, Brittania, Cephalon, Desitin, GSK, Lundbeck, Medtronic, Merck‐Serono, Novartis, Orion, Pfizer, TEVA, UCB Pharma, Valeant, and Zambon.
Maximilian Kaiser, Lynsey J. Hall, Lauritz Mildenstein, Miriam Wienecke and Olaf Gregor report no financial disclosures.
Figures
References
-
- Consky E, Basinski A, Belle L, et al. Toronto western spasmodic torticollis rating scale (TWSTRS): assessment of validity and inter‐ rater reliability. Neurology 1990;40(suppl):445.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous