Association of Rotavirus Vaccination With Inpatient and Emergency Department Visits Among Children Seeking Care for Acute Gastroenteritis, 2010-2016
- PMID: 31560386
- PMCID: PMC6777243
- DOI: 10.1001/jamanetworkopen.2019.12242
Association of Rotavirus Vaccination With Inpatient and Emergency Department Visits Among Children Seeking Care for Acute Gastroenteritis, 2010-2016
Abstract
Importance: Rotavirus vaccines have been recommended for universal US infant immunization for more than 10 years, and understanding their effectiveness is key to the continued success of the US rotavirus vaccine immunization program.
Objective: To assess the association of RotaTeq (RV5) and Rotarix (RV1) with inpatient and emergency department (ED) visits for rotavirus infection.
Design, setting, and participants: This case-control vaccine effectiveness study was performed at inpatient and ED clinical settings in 7 US pediatric medical institutions from November 1, 2009, through June 30, 2016. Children younger than 5 years seeking medical care for acute gastroenteritis were enrolled. Clinical and epidemiologic data, vaccination verification, and results of stool sample tests for laboratory-confirmed rotavirus were collected. Data were analyzed from November 1, 2009, through June 30, 2016.
Main outcomes and measures: Rotavirus vaccine effectiveness for preventing rotavirus-associated inpatient and ED visits over time for each licensed vaccine, stratified by clinical severity and age.
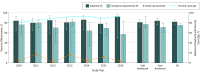
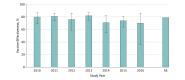
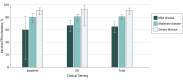
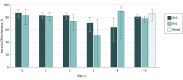
Results: Among the 10 813 children included (5927 boys [54.8%] and 4886 girls [45.2%]; median [range] age, 21 [8-59] months), RV5 and RV1 analyses found that compared with controls, rotavirus-positive cases were more often white (RV5, 535 [62.2%] vs 3310 [57.7%]; RV1, 163 [43.1%] vs 864 [35.1%]), privately insured (RV5, 620 [72.1%] vs 4388 [76.5%]; RV1, 305 [80.7%] vs 2140 [87.0%]), and older (median [range] age for RV5, 26 [8-59] months vs 21 [8-59] months; median [range] age for RV1, 22 [8-59] months vs 19 [8-59] months) but did not differ by sex. Among 1193 rotavirus-positive cases and 9620 rotavirus-negative controls, at least 1 dose of any rotavirus vaccine was 82% (95% CI, 77%-86%) protective against rotavirus-associated inpatient visits and 75% (95% CI, 71%-79%) protective against rotavirus-associated ED visits. No statistically significant difference during this 7-year period was observed for either rotavirus vaccine. Vaccine effectiveness against inpatient and ED visits was 81% (95% CI, 78%-84%) for RV5 (3 doses) and 78% (95% CI, 72%-82%) for RV1 (2 doses) among the study population. A mixed course of both vaccines provided 86% (95% CI, 74%-93%) protection. Rotavirus patients who were not vaccinated had severe infections 4 times more often than those who were vaccinated (74 of 426 [17.4%] vs 28 of 605 [4.6%]; P < .001), and any dose of rotavirus vaccine was 65% (95% CI, 56%-73%) effective against mild infections, 81% (95% CI, 76%-84%) against moderate infections, and 91% (95% CI, 85%-95%) against severe infections.
Conclusions and relevance: Evidence from this large postlicensure study of rotavirus vaccine performance in the United States from 2010 to 2016 suggests that RV5 and RV1 rotavirus vaccines continue to perform well, particularly in preventing inpatient visits and severe infections and among younger children.
Conflict of interest statement
Figures
References
-
- Cortese MM, Parashar UD; Centers for Disease Control and Prevention (CDC) . Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):-. - PubMed
-
- Linhares AC, Velázquez FR, Pérez-Schael I, et al. ; Human Rotavirus Vaccine Study Group . Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181-1189. doi:10.1016/S0140-6736(08)60524-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

