Sex-specific hippocampal metabolic signatures at the onset of systemic inflammation with lipopolysaccharide in the APPswe/PS1dE9 mouse model of Alzheimer's disease
- PMID: 31560941
- PMCID: PMC6928588
- DOI: 10.1016/j.bbi.2019.09.019
Sex-specific hippocampal metabolic signatures at the onset of systemic inflammation with lipopolysaccharide in the APPswe/PS1dE9 mouse model of Alzheimer's disease
Abstract
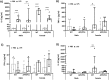
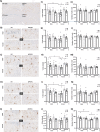
Systemic inflammation enhances the risk and progression of Alzheimer's disease (AD). Lipopolysaccharide (LPS), a potent pro-inflammatory endotoxin produced by the gut, is found in excess levels in AD where it associates with neurological hallmarks of pathology. Sex differences in susceptibility to inflammation and AD progression have been reported, but how this impacts on LPS responses remains under investigated. We previously reported in an APP/PS1 model of AD that systemic LPS administration rapidly altered hippocampal metabolism in males. Here, we used untargeted metabolomics to comprehensively identify hippocampal metabolic processes occurring at onset of systemic inflammation with LPS (100 µg/kg, i.v.) in APP/PS1 mice, at an early pathological stage, and investigated the sexual dimorphism in this response. Four hours after LPS administration, pathways regulating energy metabolism, immune and oxidative stress responses were simultaneously recruited in the hippocampi of 4.5-month-old mice with a more protective response in females despite their pro-inflammatory and pro-oxidant metabolic signature in the absence of immune stimulation. LPS induced comparable behavioural sickness responses in male and female wild-type and APP/PS1 mice and comparable activation of both the serotonin and nicotinamide pathways of tryptophan metabolism in their hippocampi. Elevations in N-methyl-2-pyridone-5-carboxamide, a major toxic metabolite of nicotinamide, correlated with behavioural sickness regardless of sex, as well as with the LPS-induced hypothermia seen in males. Males also exhibited a pro-inflammatory-like downregulation of pyruvate metabolism, exacerbated in APP/PS1 males, and methionine metabolism whereas females showed a greater cytokine response and anti-inflammatory-like downregulation of hippocampal methylglyoxal and methionine metabolism. Metabolic changes were not associated with morphological markers of immune cell activation suggesting that they constitute an early event in the development of LPS-induced neuroinflammation and AD exacerbation. These data suggest that the female hippocampus is more tolerant to acute systemic inflammation.
Keywords: APP/PS1 mouse model; Alzheimer’s disease; Hippocampus; Inflammation; Lipopolysaccharide; Metabolomics; Methionine; Microglia; Serotonin; Sex differences.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Albers E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′-methylthioadenosine. IUBMB Life. 2009;61:1132–1142. - PubMed
-
- Ashraf G.M., Tarasov V.V., Makhmutovsmall A.C.A., Chubarev V.N., Avila-Rodriguez M., Bachurin S.O., Aliev G. The possibility of an infectious etiology of Alzheimer disease. Mol. Neurobiol. 2019;56:4479–4491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

