An integrated transport mechanism of the maltose ABC importer
- PMID: 31560984
- PMCID: PMC6906923
- DOI: 10.1016/j.resmic.2019.09.004
An integrated transport mechanism of the maltose ABC importer
Abstract
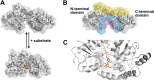
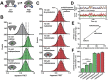
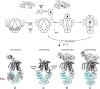
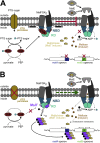
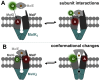
ATP-binding cassette (ABC) transporters use the energy of ATP hydrolysis to transport a large diversity of molecules actively across biological membranes. A combination of biochemical, biophysical, and structural studies has established the maltose transporter MalFGK2 as one of the best characterized proteins of the ABC family. MalF and MalG are the transmembrane domains, and two MalKs form a homodimer of nucleotide-binding domains. A periplasmic maltose-binding protein (MalE) delivers maltose and other maltodextrins to the transporter, and triggers its ATPase activity. Substrate import occurs in a unidirectional manner by ATP-driven conformational changes in MalK2 that allow alternating access of the substrate-binding site in MalF to each side of the membrane. In this review, we present an integrated molecular mechanism of the transport process considering all currently available information. Furthermore, we summarize remaining inconsistencies and outline possible future routes to decipher the full mechanistic details of transport by MalEFGK2 complex and that of related importer systems.
Keywords: ABC transporter; EPR spectroscopy; Importer; Single molecule fluorescence; Substrate-binding protein; smFRET.
Copyright © 2019 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

