Sex and chronic stress differentially alter phosphorylated mu and delta opioid receptor levels in the rat hippocampus following oxycodone conditioned place preference
- PMID: 31560995
- PMCID: PMC7768632
- DOI: 10.1016/j.neulet.2019.134514
Sex and chronic stress differentially alter phosphorylated mu and delta opioid receptor levels in the rat hippocampus following oxycodone conditioned place preference
Abstract
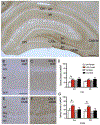
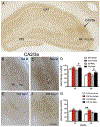
Following oxycodone conditioned place preference (CPP) in naïve female and male Sprague Dawley rats, delta- and mu-opioid receptors (DORs and MORs) redistribute in hippocampal CA3 pyramidal cells and GABAergic interneurons in a manner that would promote opioid-associative learning processes, particularly in females. MORs and DORs similarly redistribute in CA3 and hilar neurons following chronic immobilization stress (CIS) in females, but not males, essentially "priming" the opioid system for oxycodone-associative learning. Following CIS, only females acquire oxycodone CPP. The present study determined whether sex and CIS differentially affect the levels of phosphorylated MORs and DORs (pMORs and pDORs) in the hippocampus following oxycodone CPP as phosphorylation is important for opioid receptor internationalization and trafficking. In naïve oxycodone-injected (Oxy) female rats, the density of pMOR-immunoreactivity (ir) was increased in CA1 stratum oriens and CA3a,b strata lucidum and radiatum compared to saline-injected (Sal)-females. Additionally, the density of pDOR-ir increased in the pyramidal cell layer and stratum radiatum of CA2/3a in Oxy-males compared to Sal-males. In CIS females that acquire CPP, pDOR-ir levels were increased in the CA2/3a. These findings indicate only rats that acquire oxycodone CPP have activated MORs and DORs in the hippocampus but that the subregion containing activated opioid receptors differs in females and males. These results are consistent with previously observed sex differences in the hippocampal opioid system following Oxy-CPP.
Keywords: Drug addiction; Hippocampus; Learning; Opioid receptors.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Sex differences in the rodent hippocampal opioid system following stress and oxycodone associated learning processes.Pharmacol Biochem Behav. 2022 Jan;212:173294. doi: 10.1016/j.pbb.2021.173294. Epub 2021 Nov 6. Pharmacol Biochem Behav. 2022. PMID: 34752798 Free PMC article. Review.
-
Chronic immobilization stress primes the hippocampal opioid system for oxycodone-associated learning in female but not male rats.Synapse. 2019 May;73(5):e22088. doi: 10.1002/syn.22088. Epub 2019 Jan 22. Synapse. 2019. PMID: 30632204 Free PMC article.
-
Oxycodone injections not paired with conditioned place preference have little effect on the hippocampal opioid system in female and male rats.Synapse. 2021 Jan;75(1):e22182. doi: 10.1002/syn.22182. Epub 2020 Jul 29. Synapse. 2021. PMID: 32654187
-
Sex Differences in the Rat Hippocampal Opioid System After Oxycodone Conditioned Place Preference.Neuroscience. 2018 Nov 21;393:236-257. doi: 10.1016/j.neuroscience.2018.10.002. Epub 2018 Oct 11. Neuroscience. 2018. PMID: 30316908 Free PMC article.
-
The role of δ-opioid receptors in learning and memory underlying the development of addiction.Br J Pharmacol. 2015 Jan;172(2):297-310. doi: 10.1111/bph.12618. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24641428 Free PMC article. Review.
Cited by
-
An ethogram analysis of cutaneous thermal pain sensitivity and oxycodone reward-related behaviors in rats.Sci Rep. 2023 Jun 28;13(1):10482. doi: 10.1038/s41598-023-36729-6. Sci Rep. 2023. PMID: 37380739 Free PMC article.
-
Chronic stress differentially alters mRNA expression of opioid peptides and receptors in the dorsal hippocampus of female and male rats.J Comp Neurol. 2021 Jul 1;529(10):2636-2657. doi: 10.1002/cne.25115. Epub 2021 Feb 8. J Comp Neurol. 2021. PMID: 33483980 Free PMC article.
-
Sex differences in the rodent hippocampal opioid system following stress and oxycodone associated learning processes.Pharmacol Biochem Behav. 2022 Jan;212:173294. doi: 10.1016/j.pbb.2021.173294. Epub 2021 Nov 6. Pharmacol Biochem Behav. 2022. PMID: 34752798 Free PMC article. Review.
-
Neural correlates of opioid-induced risk-taking behavior in the prelimbic prefrontal cortex.J Neurosci. 2025 Mar 17;45(19):e2422242025. doi: 10.1523/JNEUROSCI.2422-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40097184
-
An ethogram analysis of cutaneous thermal pain sensitivity and oxycodone reward-related behaviors in rats.Res Sq [Preprint]. 2023 Mar 24:rs.3.rs-2679319. doi: 10.21203/rs.3.rs-2679319/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Jun 28;13(1):10482. doi: 10.1038/s41598-023-36729-6. PMID: 36993634 Free PMC article. Updated. Preprint.
References
-
- Prescription Painkiller Overdoses: A growing epidemic, especially among women. CDC Vital Signs, Centers for Disease Control and Prevention, 2013.
-
- Kesner RP, Warthen DK, Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats, Hippocampus 20 (2010) 550–557. - PubMed
-
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ, Chronic stress and neural function: accounting for sex and age, J Neuroendocrinol 19 (2007) 743–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

