Astrocyte-microglial association and matrix composition are common events in the natural history of primary familial brain calcification
- PMID: 31561281
- PMCID: PMC7317599
- DOI: 10.1111/bpa.12787
Astrocyte-microglial association and matrix composition are common events in the natural history of primary familial brain calcification
Abstract
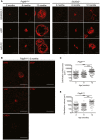
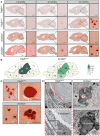
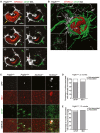
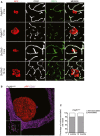
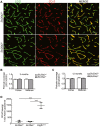
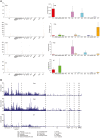
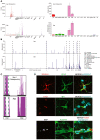
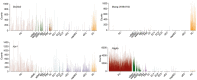
Primary familial brain calcification (PFBC) is an age-dependent and rare neurodegenerative disorder characterized by microvascular calcium phosphate deposits in the deep brain regions. Known genetic causes of PFBC include loss-of-function mutations in genes involved in either of three processes-platelet-derived growth factor (PDGF) signaling, phosphate homeostasis or protein glycosylation-with unclear molecular links. To provide insight into the pathogenesis of PFBC, we analyzed murine models of PFBC for the first two of these processes in Pdgfbret/ret and Slc20a2-/- mice with regard to the structure, molecular composition, development and distribution of perivascular calcified nodules. Analyses by transmission electron microscopy and immunofluorescence revealed that calcified nodules in both of these models have a multilayered ultrastructure and occur in direct contact with reactive astrocytes and microglia. However, whereas nodules in Pdgfbret/ret mice were large, solitary and smooth surfaced, the nodules in Slc20a2-/- mice were multi-lobulated and occurred in clusters. The regional distribution of nodules also differed between the two models. Proteomic analysis and immunofluorescence stainings revealed a common molecular composition of the nodules in the two models, involving proteins implicated in bone homeostasis, but also proteins not previously linked to tissue mineralization. While the brain vasculature of Pdgfbret/ret mice has been reported to display reduced pericyte coverage and abnormal permeability, we found that Slc20a2-/- mice have a normal pericyte coverage and no overtly increased permeability. Thus, lack of pericytes and increase in permeability of the blood-brain barrier are likely not the causal triggers for PFBC pathogenesis. Instead, gene expression and spatial correlations suggest that astrocytes are intimately linked to the calcification process in PFBC.
Keywords: PDGFB retention motive knockout; PFBC; Slc20a2 knockout; brain calcification; mass spectrometry.
© 2019 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
Michael Vanlandewijck and Christer Betsholtz are employed at the Integrated Cardio Metabolic Centre (KI), which is funded by AstraZeneca Ab. Christer Betsholtz is an advisor for AstraZeneca Ab. These affiliations do not pose any direct or indirect conflict of interest to the work described in this paper. All other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- Vetenskapsrådet/International
- FK-16-034/Forschungskredit und Stiftung für Forschung an der Medizinischen Fakultät der Universität Zürich/International
- AdG 294556 BBBARRIER/H2020 European Research Council/International
- Stiftung Synapsis - Alzheimer Forschung Schweiz AFS/International
- Knut och Alice Wallenbergs Stiftelse/International
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

