Myxozoan Adhesion and Virulence: Ceratonova shasta on the Move
- PMID: 31561529
- PMCID: PMC6843538
- DOI: 10.3390/microorganisms7100397
Myxozoan Adhesion and Virulence: Ceratonova shasta on the Move
Abstract
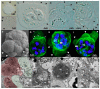
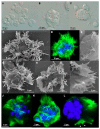
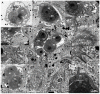
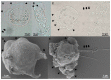
Motility factors are fundamental for parasite invasion, migration, proliferation and immune evasion and thus can influence parasitic disease pathogenesis and virulence. Salmonid enteronecrosis is caused by a myxozoan (Phylum Cnidarian) parasite, Ceratonova shasta. Three parasite genotypes (0, I, II) occur, with varying degrees of virulence in its host, making it a good model for examining the role of motility in virulence. We compare C. shasta cell motility between genotypes and describe how the cellular protrusions interact with the host. We support these observations with motility gene expression analyses. C. shasta stages can move by single or combined used of filopodia, lamellipodia and blebs, with different behaviors such as static adhesion, crawling or blebbing, some previously unobserved in myxozoans. C. shasta stages showed high flexibility of switching between different morphotypes, suggesting a high capacity to adapt to their microenvironment. Exposure to fibronectin showed that C. shasta stages have extraordinary adhesive affinities to glycoprotein components of the extracellular matrix (ECM). When comparing C. shasta genotypes 0 (low virulence, no mortality) and IIR (high virulence, high mortality) infections in rainbow trout, major differences were observed with regard to their migration to the target organ, gene expression patterns and proliferation rate in the host. IIR is characterized by rapid multiplication and fast amoeboid bleb-based migration to the gut, where adhesion (mediated by integrin-β and talin), ECM disruption and virulent systemic dispersion of the parasite causes massive pathology. Genotype 0 is characterized by low proliferation rates, slow directional and early adhesive migration and localized, non-destructive development in the gut. We conclude that parasite adhesion drives virulence in C. shasta and that effectors, such as integrins, reveal themselves as attractive therapeutic targets in a group of parasites for which no effective treatments are known.
Keywords: blebbing; cell protrusion; integrin beta; motility factors; myxozoan adhesion; rainbow trout.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
- 14-28784P/Grantová Agentura České Republiky
- 19-28399X/Grantová Agentura České Republiky
- APOSTD/2013/087/Consellería de Educación, Investigación, Cultura y Deporte, Valencia, Spain
- LM2015062/Ministry of Education, Youth and Sports of the Czech Republic
- CZ.1.05/2.1.00/01.0017/Ministry of Education, Youth and Sports of the Czech Republic
LinkOut - more resources
Full Text Sources

