Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro
- PMID: 31561750
- PMCID: PMC6764124
- DOI: 10.1186/s12974-019-1565-6
Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro
Abstract
Background: Since HIV-associated neurocognitive disorders (HANDs) occur in up to half of HIV-positive individuals, even with combined antiretroviral therapy (cART), adjunctive therapies are needed. Chronic CNS inflammation contributes to HAND and HIV encephalitis (HIVE). Baricitinib is a JAK 1/2 inhibitor approved in the USA, EU, and Japan for rheumatoid arthritis, demonstrating potent inhibition of IL-6, D-dimer, CRP, TNF-α, IFN-α/β, and other pro-inflammatory cytokines.
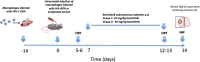
Methods: Our modified murine HAND model was used to evaluate the ability of baricitinib to cross the blood-brain barrier (BBB) and modulate monocyte/macrophage-driven HAND. Severity of HAND was measured by assessing cognitive performance of low- and high-dose baricitinib treated versus untreated HAND mice. The severity of brain neuroinflammation was evaluated in these mouse groups after flow cytometric analyses. We also assessed the ability of baricitinib to block events in myeloid and lymphoid cells in vitro that may undergird the persistence of HIV in the central nervous system (CNS) in primary human macrophages (Mϕ) and lymphocytes including HIV replication, HIV-induced activation, reservoir expansion, and reservoir maintenance.
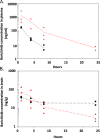
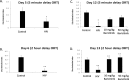
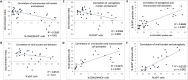
Results: In vivo, both doses of 10 and 50 mg/kg qd baricitinib crossed the BBB and reversed behavioral abnormalities conferred by HIV infection. Moreover, baricitinib significantly reduced HIV-induced neuroinflammation marked by glial activation: activated microglia (MHCII+/CD45+) and astrogliosis (GFAP). Baricitinib also significantly reduced the percentage of p24+ human macrophages in mouse brains (p < 0.05 versus HAND mice; t test). In vitro, baricitinib significantly reduced markers of persistence, reservoir size, and reseeding in Mϕ.
Conclusion: These results show that blocking the JAK/STAT pathway reverses cognitive deficits and curtails inflammatory markers in HAND in mice. Our group recently reported safety and tolerability of ruxolitinib in HIV-infected individuals (Marconi et al., Safety, tolerability and immunologic activity of ruxolitinib added to suppressive ART, 2019), underscoring potential safety and utility of JAK inhibitors for additional human trials. The data reported herein coupled with our recent human trial with JAK inhibitors provide compelling preclinical data and impetus for considering a trial of baricitinib in HAND individuals treated with cART to reverse cognitive deficits and key events driving viral persistence.
Keywords: Baricitinib; HIV-associated neurocognitive disorders; JAK inhibitor; Mononuclear phagocytes; Neuroinflammation; Object recognition testing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model.Neurobiol Dis. 2016 Aug;92(Pt B):137-43. doi: 10.1016/j.nbd.2016.02.007. Epub 2016 Feb 3. Neurobiol Dis. 2016. PMID: 26851503 Free PMC article.
-
Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors.PLoS Pathog. 2017 Dec 21;13(12):e1006740. doi: 10.1371/journal.ppat.1006740. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29267399 Free PMC article.
-
An Elvitegravir Nanoformulation Crosses the Blood-Brain Barrier and Suppresses HIV-1 Replication in Microglia.Viruses. 2020 May 20;12(5):564. doi: 10.3390/v12050564. Viruses. 2020. PMID: 32443728 Free PMC article.
-
When do models of NeuroAIDS faithfully imitate "the real thing"?J Neurovirol. 2018 Apr;24(2):146-155. doi: 10.1007/s13365-017-0601-5. Epub 2017 Dec 18. J Neurovirol. 2018. PMID: 29256039 Free PMC article. Review.
-
Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications.Brain Behav Immun. 2015 Mar;45:1-12. doi: 10.1016/j.bbi.2014.10.008. Epub 2014 Oct 22. Brain Behav Immun. 2015. PMID: 25449672 Free PMC article. Review.
Cited by
-
Antibody-drug conjugates plus Janus kinase inhibitors enable MHC-mismatched allogeneic hematopoietic stem cell transplantation.J Clin Invest. 2021 Dec 15;131(24):e145501. doi: 10.1172/JCI145501. J Clin Invest. 2021. PMID: 34730109 Free PMC article.
-
Increased IFN responses drive myeloid cell activation in people living with HIV-1.Sci Rep. 2025 Jul 1;15(1):20627. doi: 10.1038/s41598-025-04613-0. Sci Rep. 2025. PMID: 40594068 Free PMC article.
-
Macrophage polarization, inflammatory monocytes, and impaired MDSCs are associated with murine and human immune aplastic anemia.J Leukoc Biol. 2025 Jun 4;117(6):qiaf073. doi: 10.1093/jleuko/qiaf073. J Leukoc Biol. 2025. PMID: 40411822 Free PMC article.
-
Baricitinib: From Rheumatoid Arthritis to COVID-19.J Clin Pharmacol. 2021 Oct;61(10):1274-1285. doi: 10.1002/jcph.1874. Epub 2021 Jun 12. J Clin Pharmacol. 2021. PMID: 33870531 Free PMC article. Review.
-
NLRP3 Inflammasome Signaling as a Link Between HIV-1 Infection and Atherosclerotic Cardiovascular Disease.Front Cardiovasc Med. 2020 Jun 11;7:95. doi: 10.3389/fcvm.2020.00095. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32596261 Free PMC article. Review.
References
-
- Anderson AM, Munoz-Moreno JA, DR MC, Ellis RJ, Cookson D, Clifford DB, Collier AC, Gelman BB, Marra CM, JC MA, JA MC, Morgello S, Sacktor N, Simpson DM, Franklin DR, Heaton RK, Grant I, Letendre SL, Group C Prevalence and correlates of persistent HIV-1 RNA in cerebrospinal fluid during antiretroviral therapy. J Infect Dis. 2017;215:105–113. doi: 10.1093/infdis/jiw505. - DOI - PMC - PubMed
-
- Jia P, Zhao Z, Hulgan T, Bush WS, Samuels DC, Bloss CS, Heaton RK, Ellis RJ, Schork N, Marra CM, Collier AC, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, JA MC, Barnholtz-Sloan JS, Franklin DR, Rosario D, Letendre SL, Grant I, Kallianpur AR, Group CS Genome-wide association study of HIV-associated neurocognitive disorder (HAND): a CHARTER group study. Am J Med Genet B Neuropsychiatr Genet. 2017;174:413–426. doi: 10.1002/ajmg.b.32530. - DOI - PMC - PubMed
-
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

