Hearing consequences in Gjb2 knock-in mice: implications for human p.V37I mutation
- PMID: 31562289
- PMCID: PMC6782001
- DOI: 10.18632/aging.102246
Hearing consequences in Gjb2 knock-in mice: implications for human p.V37I mutation
Abstract
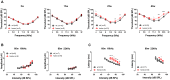
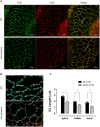
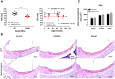
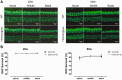
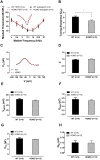
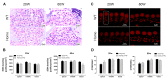
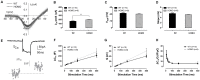
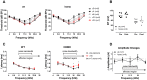
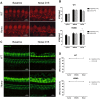
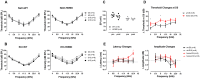
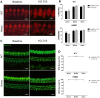
Human p.V37I mutation of GJB2 gene was strongly correlated with late-onset progressive hearing loss, especially among East Asia populations. We generated a knock-in mouse model based on human p.V37I variant (c.109G>A) that recapitulated the human phenotype. Cochlear pathology revealed no significant hair cell loss, stria vascularis atrophy or spiral ganglion neuron loss, but a significant change in the length of gap junction plaques, which may have contributed to the observed mild endocochlear potential (EP) drop in homozygous mice lasting lifetime. The cochlear amplification in homozygous mice was compromised, but outer hair cells' function remained unchanged, indicating that the reduced amplification was EP- rather than prestin-generated. In addition to ABR threshold elevation, ABR wave I latencies were also prolonged in aged homozygous animals. We found in homozygous IHCs a significant increase in ICa but no change in Ca2+ efficiency in triggering exocytosis. Environmental insults such as noise exposure, middle ear injection of KCl solution and systemic application of furosemide all exacerbated the pathological phenotype in homozygous mice. We conclude that this Gjb2 mutation-induced hearing loss results from 1) reduced cochlear amplifier caused by lowered EP, 2) IHCs excitotoxicity associated with potassium accumulation around hair cells, and 3) progression induced by environmental insults.
Keywords: GJB2; age-related hearing loss; environmental stress; hair cells; potassium recycling.
Conflict of interest statement
Figures

References
-
- Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM, Davis A, Yoshinaga-Itano C, Hind S. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ. 2001; 323:536–40. 10.1136/bmj.323.7312.536 - DOI - PMC - PubMed
-
- Zelante L, Gasparini P, Estivill X, Melchionda S, D'Agruma L, Govea N, Mila M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet. 1997; 6:1605–09. 10.1093/hmg/6.9.1605 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

