Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures
- PMID: 31562317
- PMCID: PMC6764948
- DOI: 10.1038/s41467-019-12385-1
Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures
Abstract
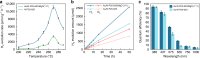
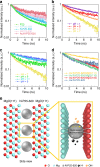
Photocatalytic water splitting is attracting enormous interest for the storage of solar energy but no practical method has yet been identified. In the past decades, various systems have been developed but most of them suffer from low activities, a narrow range of absorption and poor quantum efficiencies (Q.E.) due to fast recombination of charge carriers. Here we report a dramatic suppression of electron-hole pair recombination on the surface of N-doped TiO2 based nanocatalysts under enhanced concentrations of H+ and OH-, and local electric field polarization of a MgO (111) support during photolysis of water at elevated temperatures. Thus, a broad optical absorption is seen, producing O2 and H2 in a 1:2 molar ratio with a H2 evolution rate of over 11,000 μmol g-1 h-1 without any sacrificial reagents at 270 °C. An exceptional range of Q.E. from 81.8% at 437 nm to 3.2% at 1000 nm is also reported.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting.Inorg Chem. 2018 Apr 16;57(8):4524-4533. doi: 10.1021/acs.inorgchem.8b00283. Epub 2018 Apr 5. Inorg Chem. 2018. PMID: 29620355
-
Surface plasmon resonance enhanced direct Z-scheme TiO2/ZnTe/Au nanocorncob heterojunctions for efficient photocatalytic overall water splitting.Nanoscale. 2019 May 9;11(18):9053-9060. doi: 10.1039/c9nr01732a. Nanoscale. 2019. PMID: 31025687
-
MoS2 Quantum Dots@TiO2 Nanotube Arrays: An Extended-Spectrum-Driven Photocatalyst for Solar Hydrogen Evolution.ChemSusChem. 2018 May 25;11(10):1708-1721. doi: 10.1002/cssc.201800379. Epub 2018 May 3. ChemSusChem. 2018. PMID: 29573571
-
Understanding Charge Transport in Carbon Nitride for Enhanced Photocatalytic Solar Fuel Production.Acc Chem Res. 2019 Jan 15;52(1):248-257. doi: 10.1021/acs.accounts.8b00542. Epub 2018 Dec 31. Acc Chem Res. 2019. PMID: 30596234
-
Material Design for Photocatalytic Water Splitting from a Theoretical Perspective.Adv Mater. 2018 Nov;30(48):e1802106. doi: 10.1002/adma.201802106. Epub 2018 Oct 17. Adv Mater. 2018. PMID: 30328641 Review.
Cited by
-
Mechanism for hydrogen evolution from water splitting based on a MoS2/WSe2 heterojunction photocatalyst: a first-principle study.RSC Adv. 2020 Nov 11;10(67):41127-41136. doi: 10.1039/d0ra06939f. eCollection 2020 Nov 9. RSC Adv. 2020. PMID: 35519202 Free PMC article.
-
N-Rich Doped Anatase TiO2 with Smart Defect Engineering as Efficient Photocatalysts for Acetaldehyde Degradation.Nanomaterials (Basel). 2022 May 5;12(9):1564. doi: 10.3390/nano12091564. Nanomaterials (Basel). 2022. PMID: 35564273 Free PMC article.
-
Recent developments, advances and strategies in heterogeneous photocatalysts for water splitting.Nanoscale Adv. 2024 Jan 3;6(5):1286-1330. doi: 10.1039/d3na00442b. eCollection 2024 Feb 27. Nanoscale Adv. 2024. PMID: 38419861 Free PMC article. Review.
-
Pyro-catalysis for tooth whitening via oral temperature fluctuation.Nat Commun. 2022 Jul 29;13(1):4419. doi: 10.1038/s41467-022-32132-3. Nat Commun. 2022. PMID: 35906221 Free PMC article.
-
Light-driven oxygen evolution from water oxidation with immobilised TiO2 engineered for high performance.Sci Rep. 2021 Oct 29;11(1):21306. doi: 10.1038/s41598-021-99841-5. Sci Rep. 2021. PMID: 34716398 Free PMC article.
References
-
- Wolff CM, et al. All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods. Nat. Energy. 2018;3:862–869. doi: 10.1038/s41560-018-0229-6. - DOI
-
- Hadjiivanov KI, Klissurski DK. Surface chemistry of titania (anatase) and titania-supported catalysts. Chem. Soc. Rev. 1996;25:61–69. doi: 10.1039/cs9962500061. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

